Scroll to:
Cyclic Cushing’s syndrome: difficulties of diagnostic search and choice of treatment tactics. A clinical case
https://doi.org/10.14341/probl13140
Abstract
Cyclic Cushing’s syndrome is a pathological condition characterized by alternating periods of excessive cortisol secretion with corresponding clinical manifestations and periods of spontaneous remission of the disease.
To diagnose Cyclic Cushing’s syndrome it is necessary to record at least three episodes of excessive cortisol secretion alternating with periods of normalization of its production.
In most cases, this pathology is diagnosed in patients with ACTH-secreting pituitary tumor, however, there are rare cases of cyclic hypercorticism with ectopic ACTH secretion by tumors of different localization and without verification of pathological hormonal secretion focus. In addition, cyclic hyperproduction of cortisol can be also observed in ACTH-independent Cushing’s syndrome associated with the presence of corticosteroma or adrenal hyperplasia. The exact causes and mechanisms of the cyclic hypercorticism are currently insufficiently studied.
Due to the atypical course of the disease, the unpredictability of the occurrence of a new «cycle», the variability of its duration and manifestations (not only in different patients, but also in the same patient), verification of the diagnosis and determination of treatment tactics may be difficult in the daily practice of specialists, and the prevalence of this condition can be undervalued.
For citations:
Dzeranova L.K., Dorovskikh A.V., Pigarova E.A., Lapshina A.M., Vorotnikova S.Y., Shutova A.S., Perepelova M.A., Grigoriev A.Yu., Azizyan V.N. Cyclic Cushing’s syndrome: difficulties of diagnostic search and choice of treatment tactics. A clinical case. Problems of Endocrinology. 2023;69(1):8-14. https://doi.org/10.14341/probl13140
RELEVANCE
A cyclic form of hypercorticism was first reported in the late 1950s [1]; however, intermittent Cushing’s syndrome has even to this day remained a focus area for researchers.
Possible causes of this condition have been identified, including hypothalamus dysfunction, pathological response of corticotrophs to neurotransmitters (such as corticoliberin, dopamine, neuroepinephrine, serotonin, or g-amino butyrate), spontaneous pituitary apoplexy causing disruption of corticotroph function, pathology of reverse feedback regulation in the hypothalamo-pituitary-adrenal axis, and more. The exact mechanisms triggering hypercorticism periods followed by periods of normal hormone secretion remain elusive [2].
Various sources indicate that cyclic Cushing’s syndrome occurs in 15% to 36% of patients with endogenous hypercorticism [3]. Variable frequency and duration of phases of this disease (ranging from several hours to several months); unpredictability of any new hypercorticism “cycle” or any new spontaneous hormonal status normalisation; diversity of clinical manifestations and their extent — all these factors make cyclic Cushing’s syndrome one of the most serious and pressing issues in contemporary endocrinology.
This paper reports a case of cyclic Cushing’s syndrome, discusses the challenges associated with diagnosis determination and treatment tactics, and summarises the findings.
CLINICAL CASE
Patient S., a 57-year-old female
Since November 2017 she noticed some changes in her appearance in the form of facial swelling and weight gain without changing her usual diet. Both initially and during the weight gain her body mass index (BMI) was normal; her medical history contained no data on any previous carbohydrate metabolism disorders or arterial hypertension (AH). The location of fat deposits predominantly in the facial and abdomen areas was unusual. In addition, the patient reported neurological problems (limb numbness, impaired coordination), so she consulted a neurologist. A brain MRI in 2018 revealed a 9×6×6-mm pituitary microadenoma (Figure 1). No hormonal tests for neoplasm activity were performed nor any specific treatment prescribed.
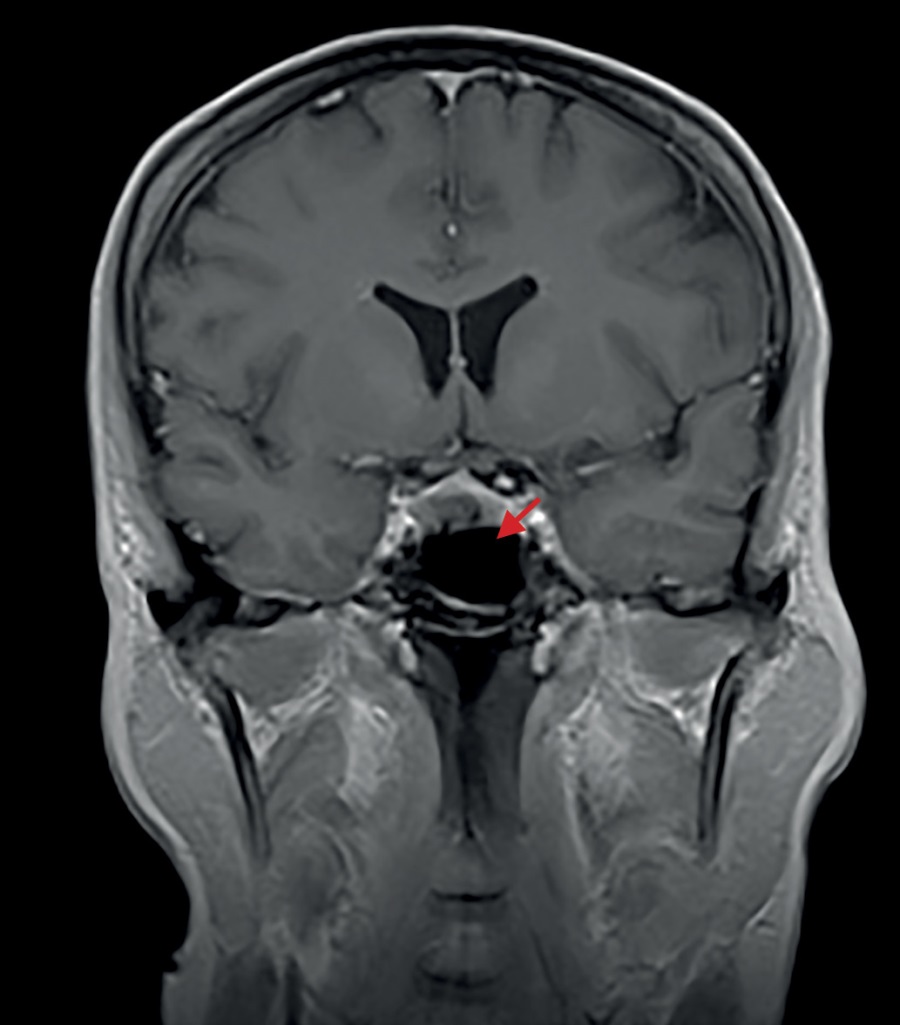
Figure 1. The 2018 brain MRI image made prior to surgical intervention (transnasal adenomectomy).
In the following six months, her condition worsened: she developed lower limb cramps, substantial weakness and fatigue and had episodes of hypertension up to 160/100 mmHg. In May 2018, a laboratory examination found elevated morning adrenocorticotropic hormone (ACTH) level (145 pg/ml vs. the normal range of up to 46.0 pg/ml) and no cortisol suppression during the small dexamethasone test (serum cortisol at 548.8 nm/L). Large dexamethasone test found a reduction in cortisol by more than 50%. Taking into account these findings and the already diagnosed pituitary adenoma, she was diagnosed with endogenous hypercorticism of central genesis. In addition, an abdominal CT imaging was made (findings: no pathology). A transnasal adenomectomy (not followed by any histological or immunohistochemical tests) was performed at her local clinic in September 2018. Post-surgery, the patient reported muscle weakness and general weakness; a 5-mg/day hydrocortisone therapy was initiated. At control examination one month after the surgery, the morning serum ACTH was at 50.0 pg/ml (vs. the normal range of up to 46.0 pg/ml); two months post-surgery it was at 31.0 pg/ml, and morning cortisol at 146 nmol/L. Three months post-surgery, given no clinical or laboratory signs of adrenal insufficiency, the glucocorticoid therapy was discontinued, while the patient reported a general improvement of her condition.
In June 2020, the patient underwent a severe emotional stress, following which she again reported a “moon-shaped face”, a significant increase in appetite, a weight gain of 8 kg over six months without any change in diet, and substantial weakness. Laboratory tests identified a hypercorticism recurrence: saliva cortisol taken 11 pm was at 69 nmol/L (normal range: 0.5 to 9.65); cortisol in day’s urine was at 2,433.0 nmol/day (normal range: 100 to 379); morning serum ACTH was at 166 pg/ml (normal range: 2 to 25.5); cortisol was at 712 nmol/L (normal range: 64 to 327). Brain MRI made in July 2020 (Figure 2) identified post-surgery changes in the sellar area; no 2018 data to compare against were available. No specific treatment was prescribed, and she was advised to consult the Endocrinology Research Centre (Moscow, Russia) where she was examined in September 2020. The tests found a regression of laboratory signs of hypercorticism: saliva cortisol taken 11 pm was only slightly elevated (10.28 mmol/L vs. the normal range of up to 9.65 nmol/L); cortisol in day’s urine was within the reference range; the small dexamethasone test was positive: cortisol at 45.0 nmol/L. No evidence of carbohydrate metabolism disorder was found (serum glucose at 4.68 mmol/L). An X-ray densitometry found no significant changes. External manifestations of endogenous hypercorticism had ceased, as did the complaints typical for this disease. Despite the lack of a full laboratory remission, no repeat surgery was recommended, as the patient’s clinical condition had improved. A follow-up was recommended.
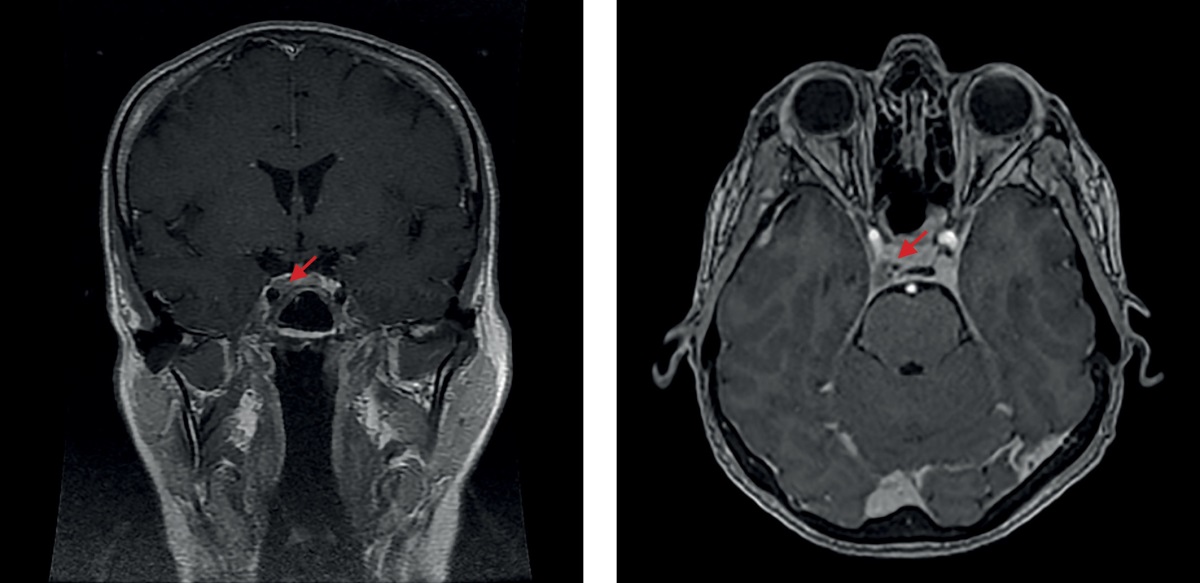
Figure 2. The 2020 brain MRI image made after a transnasal adenomectomy
In July 2021, the patient was infected with Covid-19 and developed moderate symptoms of the disease. In August 2021, her condition worsened once again, as she felt craving for food, gained weight (by 7 kg in three months), and experienced sleep disturbances, general weakness, limb numbness, and pain in her joints. Laboratory tests revealed the underlying disease decompensation (saliva cortisol taken 11 pm was at 27.0 nmol/L; morning ACTH was at 90 pg/ml; cortisol during the small dexamethasone test was at 596 nmol/L). In November 2021 she was readmitted to the Endocrinology Research Centre (Moscow, Russia) where tests showed cortisol in evening saliva at 13.46 nmol/L (normal range: 0.5 to 9.65); cortisol in day’s urine at 398 nmol/day (normal range: 100 to 379); ACTH rhythm (morning/evening) at 63.55/36.65 pg/ml. Contrast-enhanced brain MRI imaging (Figure 3) revealed a 6×9×11-mm pituitary macroadenoma with endo- and laterosellar extension to the right-hand side. It was Knosp grade 4 (definite invasion as the tumour completely surrounded C4 of the internal carotid artery). An X-ray measurement of bone mineral density (BMD) verified osteopenia in the lumbar spine (minus 1.3, T-criterion) and in the left femur (-1.0 T-criterion), as well as osteoporosis of the distal third of the left radius (minus 3.1, T-criterion). Taking into account the tumour size and growth, as well as the lack of a complete laboratory remission at the time of clinical remission, a case conference attended by neurosurgery department head decided to follow up the patient and to perform a transnasal transsphenoidal adenomectomy, given a clear trend of the underlying disease towards development.
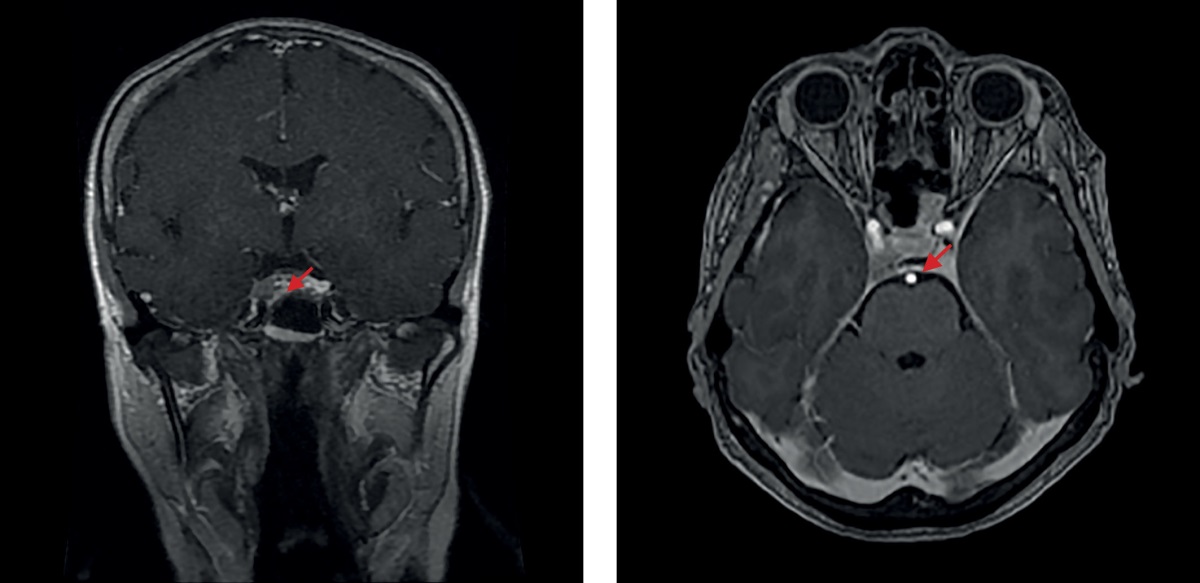
Figure 3. The 2021 brain MRI image made after a transnasal adenomectomy
From January 2022, she was again reporting bad condition and developing signs of hypercorticism. Outpatient clinic tests found: cortisol in day’s urine at 2,664.0 nmol/day; ACTH at 123 pg/ml; serum cortisol at 940 nmol/L, saliva cortisol taken 11 pm at 29.9 nmol/L. In March 2022, she was admitted to the Endocrinology Research Centre (Moscow, Russia); laboratory tests confirmed a relapse of endogenous hypercorticism: free urine cortisol at 2,557.6 nmol/day (normal range: 100 to 379); saliva cortisol taken 11 pm at 17.52 nmol/L (normal range: 0.5 to 9.65); evening blood cortisol at 704.4 nmol/L (normal range: 64 to 327); morning ACTH at 102.8 pg/ml (normal range: 7.2 to 63.3); evening ACTH at 71.7 pg/ml (normal range: 2 to 25.5). Brain MRI (Figure 4) revealed a 8×12×10-mm (slightly greater vs. the image made on 20 November 2021) Knosp-4 pituitary adenoma with endo- and laterosellar extension to the righthand side. On 29 March 2022, the patient underwent another surgical treatment, a transnasal transsphenoidal adenomectomy. Immunohistochemical tests of the removed tissue found densely granular corticotropinoma (Ki-67 expression was not measured). Post-surgery, the patient has felt well and had no major complaints.
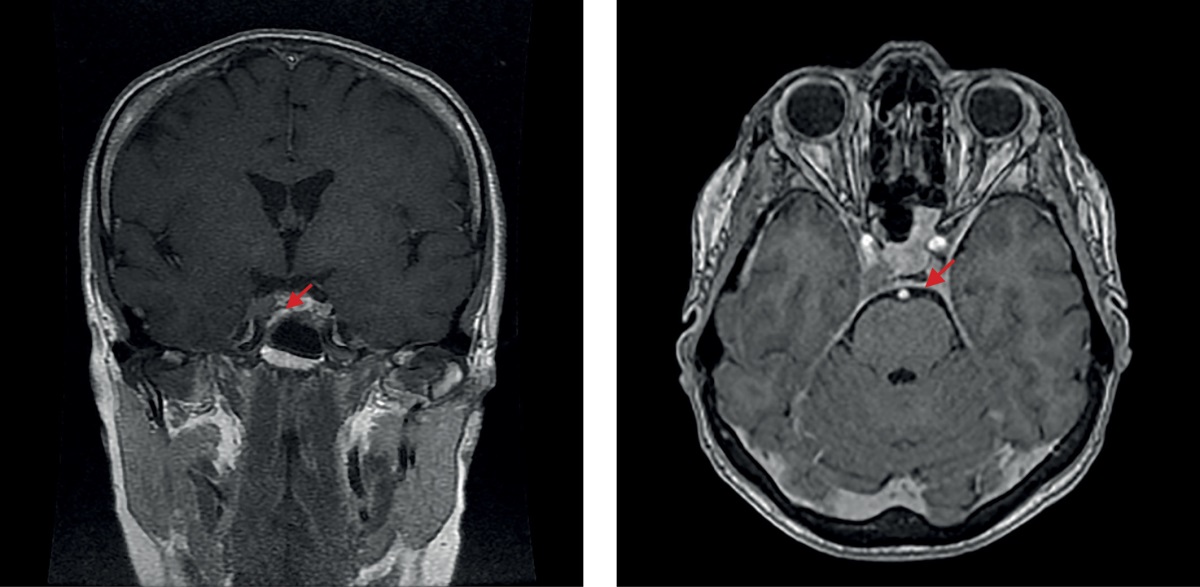
Figure 4. The 2022 brain MRI image prior to the second surgical intervention (a repeat transnasal
DISCUSSION
Most often, the pattern of the disease caused by excessive endogenous cortisol is specific and consistent. The main manifestations of long-term hypercorticism are: a characteristic redistribution of adipose tissue, elevated blood pressure, systemic osteoporosis, sexual dysfunction, immunodeficiency, skin trophism disorders, myopathy, and carbohydrate metabolism disorder [3]. The above case is characterised by cycles wherein cortisol hyper-secretion episodes were followed by periods of hormone profile normalisation and vice versa; clinical manifestations were transitory and quite non-specific, so the diagnosis was not entirely clear.
A common idea is that cyclic Cushing’s syndrome is to be verified by observing at least three peaks and two dips in cortisol secretion [2]. In the above case, as many as four laboratory-recorded hypercorticism “cycles” were observed, leaving no doubt that intermittent endogenous hypercorticism should be diagnosed.
Notably, just as is the case with the classical endogenous hypercorticism, cyclic hypercortisolemia can be caused by hormonal activity of a corticotropinoma (in 80% to 85% of cases), or more seldom by ectopic corticotropin release or by other causes independent of ACTH secretion (in adenoma or adrenal cortex carcinoma, bilateral macro- or micro-nodular hyperplasia) [5].
If ACTH-dependent hypercorticism diagnosis is verified through laboratory tests, any further treatment tactics should be determined after identifying the source of abnormal ACTH secretion. ACTH-secreting pituitary adenoma usually follows negative feedback mechanism, as demonstrated by a reduction of cortisol levels by more than 50% of baseline during high-dose glucocorticoid therapy (a large dexamethasone test); ACTH-ectopic tissue does not behave that way. However, this diagnostic method has quite low sensitivity, as in patients with pituitary macroadenoma whose pathological tissue is completely autonomous the ACTH secretion by pituitary adenoma will not necessarily be suppressed by high-dose glucocorticoid therapy [6]. Moreover, in some cases ACTH secretion by ectopic neoplasms is inhibited by high-dose glucocorticoid therapy through the negative feedback mechanism [7].
Reports of a more severe course of ACTH-ectopic syndrome can be found in the literature, but such manifestations are not specific. High evening ACTH (over 110 pg/ml) occurs more often in patients with ectopic source of ACTH secretion (70% of cases) [8]; however, this laboratory indicator is likewise ambiguous.
Brain MRI is recommended for topical diagnosis, and in most cases it reveals a pituitary adenoma. Pituitary adenoma over 6 mm in size combined with endogenous hypercorticism can be diagnosed as Itsenko–Cushing’s disease, whereas a neoplasm smaller than 6 mm in diameter is not always indicative of a source of excessive ACTH secretion. Literature suggests that pituitary incidentaloma of this size can be found in 10% to 20% of healthy individuals [9][10]. Studies have shown a 14.4% incidence of pituitary incidentaloma on autopsy and a 22.5% incidence on MRI screening [11]. In the above case, MRI imaging confirmed from the start a pituitary adenoma over 6 mm; thus, Itsenko– Cushing’s disease could be diagnosed and surgical treatment selected as a priority option. Moreover, a positive large dexamethasone test and the lack of ACTH secretion rhythm abnormality indirectly confirmed the diagnosis.
If no hypothalamic-pituitary pathology is suspected, additional instrumental examination will be required. An ectopic source of cyclic ACTH secretion may be located in different areas: in the lungs, thymus, pancreas, thyroid, adrenal gland, ovary, kidney, stomach, or appendix; it can be either benign or malignant [12][13]. Rare cases of ACTH-secreting paraganglioma, neuroblastoma, and chemodectoma have also been reported [14]. Therefore, when ACTH-ectopic syndrome is suspected, identifying the source of pathological ACTH secretion may be difficult and even more so in a cyclic course of the disease.
Instrumental diagnostic techniques include chest, mediastinal, abdominal and pelvic CT imaging [15]. If CT imaging yields no findings, it is recommended to consider somatostatin receptors scintigraphy with ¹¹¹In-octreotide, 123I-Metaiodobenzylguanidine or 99mTc tectrotide and/or combined positron emission tomography (PET) and CT with DOTA conjugated radiopharmaceuticals (68Ga-DOTA-TATE) [4].
Currently, the most informative method for differential diagnosis of ACTH-secreting pituitary adenoma and ACTH-ectopic syndrome is bilateral single-stage selective blood sampling from inferior petrosal sinuses (sensitivity: 88% to 100%) [16][17]. In the above case, no indication for such invasive test was identified, and a repeat transnasal adenomectomy was warranted by a recurrence of the underlying disease, as confirmed by instrumental examination findings (brain MRI: pituitary macroadenoma) and by the lack of neoplasm growth through the follow-up period.
Thus, the choice of treatment for cyclic Cushing’s syndrome depends on the location of the pathological secretion source. Surgery is considered to be the most effective and preferable treatment if corticotropinoma has been identified. The success of surgical treatment depends on many factors: tumour size and cellular structure, degree of its invasion into the cavernous sinuses, and the neurosurgeon’s dexterity. Various sources suggest that transnasal adenomectomy leads to remission in 65% to 90% of cases. However, a relapse of the disease cannot be ruled out.
Development of adrenal insufficiency indicates a remission of the underlying disease. In cyclic hypercorticism, the challenge is that post-surgery laboratory test data may be similar to those corresponding to the end of a hypercorticism “cycle”; thus, a long-term follow-up is necessary.
If the disease persists after the surgical treatment or if it is impossible to achieve a complete removal of pituitary tumour tissue, several options for further treatment should be considered: a repeat surgery; radiotherapy and/or continuous pharmacotherapy. The issue should be decided by a multidisciplinary medical team on a case-by-case basis.
Thus, radiotherapy is an effective treatment if pituitary adenoma cannot be entirely removed or in Cushing’s disease relapse. Stereotactic radiosurgery and fractionated external radiotherapy are the most common methods. It should be taken into account that the benefits of this treatment may be delayed (by several months or years), while the risk of hypopituitarism is significant [5].
As to pharmacological therapy, international studies have described the efficiency of many drugs, such as steroidogenesis inhibitors (metyrapone, ketoconazole, mitotane, etomidate, osilodrostat), drugs affecting glucocorticoid receptors (mifepristone), and drugs affecting pituitary gland (cabergoline, pasireotide) [5]. It is important that, among these drugs, only pasireotide has registered indications for use in Russia to control endogenous hypercorticism. The possibility of using the other drugs, even though it may be warranted from pathophysiological point of view, must be discussed with patients individually [4].
In cases of an ectopic ACTH secretion source, surgical removal should be considered. If surgical treatment is not possible (e.g., the source of ACTH secretion is not clearly visible, or a metastatic spread has occurred), pharmacological therapy (by somatostatin analogues, steroidogenesis blockers, etc.) should be considered [5][18].
In particularly severe condition and/or if the above methods have not been sufficiently effective, and the location of the ACTH secretion source is uncertain, bilateral adrenalectomy remains a potential life-saving treatment option [2].
As the signs and symptoms of both classical and cyclic endogenous hypercorticism tend to develop over time and the disease can ultimately be fatal, the treatment of this pathological condition should not be delayed.
CONCLUSION
Cyclic Cushing’s syndrome is a rare form of endogenous hypercorticism, which is difficult to diagnose due to inconsistent clinical manifestations and incoherent laboratory findings. The onset and duration of any new hypercorticism episode are unpredictable; the duration of inter-cycle periods may vary significantly (from several days to several months). The genesis of the disease can be central (in most cases) or peripheral, making it even more difficult to verify the diagnosis and select a further treatment strategy.
The above clinical case presents four documented episodes of hypercorticism followed by periods of normalisation, thus suggesting a cyclic form of Cushing’s disease. Given the MRI verification of a pituitary adenoma over 6 mm in size, surgical treatment in the form of transnasal adenomectomy was warranted. Post-surgery development of adrenal insufficiency signalled the success of the surgical treatment. However, the disease recurrence as confirmed by clinical and laboratory data necessitated further treatment. A repeat surgical procedure was warranted by disease regression and pituitary macroadenoma (as per a brain MRI test), as well as availability of a highly specialised in-patient clinic. Post-surgery laboratory tests revealed development of adrenal insufficiency, regression of clinical manifestations, and general improvement, thereby pointing to the treatment effectiveness. An immunohistochemical test of the removed tissue confirmed corticotropinoma. At present, the patient is followed up as an outpatient.
Thus, cyclic Cushing’s syndrome is a diagnostic dilemma that physicians may encounter in clinical practice. It requires an especially diligent patient follow-up and careful evaluation of clinical and laboratory data. Reports of clinical cases of cyclic hypercorticism are important as they contribute to a deeper understanding of the specifics of this disease and the diagnostic and treatment options.
As the number of patients with overweight or obesity is increasing, clinicians need to be even more vigilant when hypercorticism may be suspected based on medical history, particularly where tests yield conflicting results. If in doubt, repeat laboratory and instrumental tests should be performed to rule out this pathology and to identify individual symptoms of hypercorticism, lest this rare but severe pathology be overlooked.
DISCLOSURES
Funding source. This study was self-initiated by the authors and no funding was raised.
Conflict of interest. The authors hereby declare no actual or potential conflict of interest related to this publication. Every author has approved the final version of the text prior to publication and agreed to accept responsibility for all aspects of this study, which implies due investigation and resolution of any issue related to the accuracy or integrity of any part thereof.
Patients’ consent. All patients referred to in this study provided their written consent to having their medical data published in Problems of Endocrinology.
References
1. Birke G, Diczfalusy E. Fluctuation in the excretion of adrenocortical steroids in a case of Cushing’s syndrome. J Clin Endocrinol Metab. 1956;16(2):286-290. doi: https://doi.org/10.1210/jcem-16-2-286
2. Świątkowska-Stodulska R, Berlińska A, Stefańska K, et al. Cyclic Cushing’s Syndrome – A Diagnostic Challenge. Front Endocrinol (Lausanne). 2021;12(9):1158-1168. doi: https://doi.org/10.3389/fendo.2021.658429
3. Albani A, Berr CM, Beuschlein F, et al. A pitfall of bilateral inferior petrosal sinus sampling in cyclic Cushing’s syndrome. BMC Endocr Disord. 2019;19(1):105. doi: https://doi.org/10.1186/s12902-019-0433-9
4. Rossiiskaia assotsiatsiia endokrinologov. Klinicheskie rekomendatsii: Bolezn’ Itsenko-Kushinga. Moscow; 2021. (In Russ.).
5. Albiger NME, Scaroni CM, Mantero F. Cyclic Cushing’s syndrome: an overview. Arq Bras Endocrinol Metabol. 2007;51(8):1253-1260. doi: https://doi.org/10.1590/S0004-27302007000800011
6. Liu Z, Zhang X, Wang Z, et al. High positive predictive value of the combined pituitary dynamic enhanced MRI and high-dose dexamethasone suppression tests in the diagnosis of Cushing’s disease bypassing bilateral inferior petrosal sinus sampling. Sci Rep. 2020;10(1):14694. doi: https://doi.org/10.1038/s41598-020-71628-0
7. Araya AV, Romero C, Lemp M. Combined dexamethasone and desmopressin test in the differential diagnosis of ACTH-dependent Cushing’s syndrome and pseudo-cushing’s states. Pituitary. 2017;20(5):602-603. doi: https://doi.org/10.1007/s11102-017-0824-8
8. Belaia ZhE. Ranniaia diagnostika endogennogo giperkortitsizma. Kanonicheskii signal’nyi put’ i izmenenie kostnogo metabolizma pri gliukokortikoidnom osteoporoze [dissertation]. Moscow; 2013. (In Russ.).
9. Hall WA, Luciano MG, Doppman JL, et al. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med. 1994;120(10):817-820. doi: https://doi.org/10.7326/0003-4819-120-10-199405150-00001
10. Coulon G, Fellmann D, Arbez-Gindre G. Pageaut Latent pituitary adenoma. Autopsy study. 1983;59(40):2747-2750.
11. Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas. Cancer. 2004;101(3):613-619. doi: https://doi.org/10.1002/cncr.20412
12. Miao H, Lu L, Zhu H, et al. Experience of Ectopic Adrenocorticotropin Syndrome: 88 Cases With Identified Causes. Endocr Pract. 2021;27(9):866-873. doi: https://doi.org/10.1016/j.eprac.2021.02.015
13. Isidori AM, Lenzi A. Ectopic ACTH syndrome. Arq Bras Endocrinol Metabol. 2007;51(8):1217-1225. doi: https://doi.org/10.1590/S0004-27302007000800007
14. Farage M, Costa MA da DL, Godoy-Matos AF. A rare case of Cushing syndrome by cyclic ectopic-ACTH. Arq Bras Endocrinol Metabol. 2012;56(5):324-330. doi: https://doi.org/10.1590/S0004-27302012000500008
15. Vilar L, Freitas MDC, Faria M, et al. Pitfalls in the diagnosis of Cushing’s syndrome. Arq Bras Endocrinol Metabol. 2007;51(8):1207-1216. doi: https://doi.org/10.1590/S0004-27302007000800006
16. Dedov II, Belaya ZE, Sitkin II, et al. Significance of the method of selective blood collection from the inferior petrosal sinuses for differential diagnosis of ACTH-dependent hypercorticism. Problems of Endocrinology. 2009;55(6):35-40. (In Russ.). doi: https://doi.org/10.14341/probl200955635-40
17. Suwaifi Y, Al Zaman Y. Cyclical Cushing’s syndrome: A Diagnostic dilemma. Bahrain Medical Bulletin. 2019;41(2):106-108.
18. Isidori AM, Kaltsas GA, Pozza C, et al. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab. 2006;91(2):371-377. doi: https://doi.org/10.1210/jc.2005-1542
About the Authors
L. K. DzeranovaRussian Federation
Larisa K. Dzeranova - MD, PhD.
Moscow
Competing Interests:
None
A. V. Dorovskikh
Russian Federation
Anna V. Dorovskikh – MD.
11 Dm. Ulyanova street, 117036 Moscow
Competing Interests:
None
E. A. Pigarova
Russian Federation
Ekaterina A. Pigarova - MD, PhD.
Moscow
Competing Interests:
None
A. M. Lapshina
Russian Federation
Anastasia M. Lapshina - MD, PhD.
Moscow
Competing Interests:
None
S. Y. Vorotnikova
Russian Federation
Svetlana Y. Vorotnikova - MD, PhD.
Moscow
Competing Interests:
None
A. S. Shutova
Russian Federation
Aleksandra S. Shutova - MD.
Moscow
Competing Interests:
None
M. A. Perepelova
Russian Federation
Margarita A. Perepelova.
Moscow
Competing Interests:
None
A. Yu. Grigoriev
Russian Federation
Andrei Yu. Grigoriev - MD, PhD.
Moscow
Competing Interests:
None
V. N. Azizyan
Russian Federation
Vilen N. Azizyan - MD, PhD.
Moscow
Competing Interests:
None
Supplementary files
|
|
1. Figure 1. MRI of the brain before surgery (transnasal adenomectomy), 2018 | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(127KB)
|
Indexing metadata ▾ | |
|
|
2. Figure 2. Brain MRI after transnasal adenomectomy, 2020 | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(218KB)
|
Indexing metadata ▾ | |
|
|
3. Figure 3. Brain MRI after transnasal adenomectomy, 2021 | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(192KB)
|
Indexing metadata ▾ | |
|
|
4. Figure 4. MRI of the brain before the second operation (repeated transnasal adenomectomy), 2022 | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(243KB)
|
Indexing metadata ▾ | |
Review
For citations:
Dzeranova L.K., Dorovskikh A.V., Pigarova E.A., Lapshina A.M., Vorotnikova S.Y., Shutova A.S., Perepelova M.A., Grigoriev A.Yu., Azizyan V.N. Cyclic Cushing’s syndrome: difficulties of diagnostic search and choice of treatment tactics. A clinical case. Problems of Endocrinology. 2023;69(1):8-14. https://doi.org/10.14341/probl13140

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0).









































.jpg)


