Scroll to:
Thyrotropin-secreting pituitary adenomas: clinical features and results of treatment in 45 patients
https://doi.org/10.14341/probl13325
Abstract
BACKGROUND: Thyrotropin-secreting pituitary adenomas (TSH-PA) are a rare cause of thyrotoxicosis and account for 0.5-2% of all pituitary adenomas. Taking into account the rarity of the disease, it is extremely important to analyze each case of TSH-PA. AIM: To analyze the clinical characteristics and treatment outcomes of patients with TSH-PA, as well as to determine preoperative and early postoperative factors that predict long-term remission.
MATERIALS AND METHODS: In a single-center retrospective study we analyzed clinical signs, laboratory and instrumental studies, as well as the treatment outcomes of patients with TSH-PA from 2010 to 2023. Preoperative factors, as well as TSH level measured on day 3 postoperatively, were evaluated for their ability to predict long-term remission when comparing groups of patients with and without remission. RESULTS: The study included 45 patients with TSH-PA (14 men, 31 women), with a median age of 45 years [30; 57]. The most common clinical manifestations of TSH-PA were: cardiac arrhythmia in 37 (82.2%) patients, thyroid pathology in 27 (60%), neurological disorders in 24 (53.35%). Most PAs were macroadenomas (n=35, 77.8%). Preoperatively, 28 (77.8%) patients received somatostatin analogs, and 20 (71.4%) patients were euthyroid at the time of surgery. Surgical treatment was performed in 36 (80%) patients, postoperative remission was achieved in 31 cases (86.1%). Administration of somatostatin analogues to patients with no remission/relapse after surgery lead to the remission in 100% of cases (4/4). A 1 mm increase in PA size raised the odds of recurrence/no remission by 1.15-fold,and PA invasion during surgery — by 5.129 fold. A TSH level on day 3 postoperatively above 0.391 mIU/L (AUC, 0.952; 95% CI 0.873–1.000; standard error 0.04; p<0.001) identifies patients with relapse/absence of remission after surgical treatment (sensitivity = 100%, specificity = 88.9%).
CONCLUSION: The TSH-PA in the structure of PAs is extremely rare, and as a result, most of them are misdiagnosed and detected already at the stage of macroadenoma. The most effective method of treatment is transnasal transsphenoidal adenomectomy. Somatostatin analogues can be used as second-line therapy if surgical treatment is ineffective. We have proposed a possible model for postoperative TSH levels (>0.391 mU/l) to predict recurrence of TSH-PA, which requires validation on an expanded number of cases.
For citations:
Trukhina D.A., Przhiyalkovskaya E.G., Belaya Zh.E., Grigoriev A.Yu., Azizyan V.N., Mamedova E.O., Rozhinskaya L.Ya., Lapshina A.M., Pigarova E.A., Dzeranova L.K., Platonova N.M., Troshina E.A., Melnichenko G.A. Thyrotropin-secreting pituitary adenomas: clinical features and results of treatment in 45 patients. Problems of Endocrinology. 2024;70(2):23-36. https://doi.org/10.14341/probl13325
INTRODUCTION
Thyrotropin-secreting pituitary adenomas (TSH-PA) account for 0.5–2% of all pituitary adenomas and are a rare cause of thyrotoxicosis [1]. The vast majority of TSH-PAs are benign tumours and only three TSH-producing carcinomas have been described in the literature [2]. The first case of TSH-PA was documented by Jailer J.W. and Holub D.A. in 1960; they suggested and proved that thyrotoxicosis syndrome could be associated with PA [3]. In total, about 540 patients with TSH-PA have been described in the worldwide literature [4][5]; 29 cases have been reported in the Russian literature [6][7][8]. TSH-secreting adenomas may also be part of hereditary syndromes such as multiple endocrine neoplasia type 1 syndrome (mutations in MEN1 gene); in addition, 2 cases with a mutation in AIP gene (Familial isolated pituitary adenoma) have been described [9][10]. Most (~70%) TSH-PAs secrete only TSH; the remaining 30% are mixed PAs, the most common combination of which is co-secretion with growth hormone (GH) or prolactin (PRL) [9].
Previously, TSH-PA was diagnosed at the stage of invasive macroadenoma and was considered difficult to treat. However, the introduction of new methods of immunometric analysis to assess thyroid function and high-resolution instrumental diagnostics has significantly improved the diagnosis of patients with thyrotoxicosis, allowing more frequent detection of central thyrotoxicosis [1]. TSH-PA should be suspected when an inappropriate TSH level (normal or elevated) is found in individuals with elevated thyroid hormone levels. To identify central thyrotoxicosis, repeated laboratory testing should always be performed to exclude laboratory errors and transient changes in thyroid hormone levels. Next, highly sensitive functional tests (thyrotropin-releasing hormone test, triiodothyronine suppression test, measurement of α-subunit/TSH molar ratio, search for mutations in THRB gene [1][5][11]) can be used to identify TSH-PAs and to make a differential diagnosis with thyroid hormone resistance syndrome. In some cases, TSH-PA can also be diagnosed by trial therapy with long-acting somatostatin analogues (SAs) for at least two months [9][12] and short-acting SAs [13]. Despite all the above, as De Herdt S. et al. have pointed out, pituitary macroadenomas are found on imaging studies in most patients at the time of diagnosis of TSH-PA, although a steady decrease in the frequency of such macroadenomas has been reported [5][14][15][16].
The time taken to make the correct diagnosis may be due to the subtle clinical picture of thyrotoxicosis, and in macroadenomas neurological symptoms may be more prominent [1]. Measuring TSH levels alone to exclude functional thyroid disorders may also lead to misdiagnosis and «missing» cases of central thyrotoxicosis, as normal TSH levels with elevated free thyroxine (free T4) and/or triiodothyronine (free T3) fractions are common [17]. In Russia, the diagnosis of TSH-PA in complex situations is difficult due to the unavailability of the thyrotropin-releasing hormone triiodothyronine [6].
Transsphenoidal adenomectomy is considered the first-line treatment for TSH-PA. Meta-analysis shows that biochemical remission after surgery occurs in 69.7% of patients (95% confidence interval (CI) 61.1%-78.4%); although in some studies the postoperative remission rate is 100%, it is generally lower than desired [4]. Preoperative administration of SAs may contribute to the reduction of PA size due to the expression of somatostatin receptors (SSTR) by TSH-PAs [16]. However, there is still no consensus on the need for neoadjuvant SA therapy, as it does not increase the rate of radical tumour removal as well as the association of preoperative euthyroid status with surgical outcomes [5]. When surgery is ineffective, refused or impossible, SAs can be prescribed to control the disease: postoperative use of SAs leads to disease stabilisation in 81.9% of cases [5][16].
The use of dopamine receptor agonists (DRAs), especially cabergoline, is effective mainly in PAs with mixed secretion – PRL+ TSH. DRA treatment as monotherapy for TSH-PA yields contradictory results [1][18]. When SSTR and dopamine D2 receptor (D2R) expression occurs in distant tumour tissue, co-administration of SAs and DRAs may be generally effective for disease control when surgery is unsuccessful [4].
Due to the high efficacy of SAs and the risk of hypopituitarism and other complications following radiotherapy, this treatment option is currently used much less frequently. However, in the case of ineffective surgery or drug treatment and aggressive tumour growth, radiotherapy followed by the administration of SA is an option [4][5][9].
Cure criteria for TSH-PA patients have not been clearly established due to the rarity of the disease and the wide heterogeneity of methods used to diagnose remission. An undetectable TSH level one week after surgery may indicate complete tumour resection, provided that no other treatment methods have been used prior to surgery [19]. In a study by Kim S.H. et al, the TSH level 12 hours after surgery was found to be the strongest predictor of remission with a threshold of 0.62 μME/mL [20]. Normalisation of the α-subunit/TSH molar ratio is also generally a good indicator of treatment response; however, its sensitivity is quite low, as this ratio is normal in approximately 25% of TSH-PA patients. The most sensitive and specific test for confirming complete adenoma removal, in the absence of contraindications, remains the T3 test [21]; therefore, if functional tests are not available, other methods must be found to diagnose long-term remission in TSH-PA patients.
The data collected on TSH-PA are extremely relevant, and each case can contribute to increasing awareness of the disease and clinical experience in global practice. The aim of our study was to analyse the clinical features and specifics of diagnosis in patients when functional tests are not available, the particularities of treatment, remission criteria and follow-up in 45 TSH-PA patients followed at the same institution.
METHODS
Design of the study
A single-centre observational retrospective study.
Inclusion criteria
PA patients with normal or elevated blood TSH combined with elevated free T4 and/or free T3 level; patients with IHC-confirmed TSH-PA diagnosis.
Setting
Patients were seen at the Endocrinology Research Centre (Moscow, Russia) during the period 2010–2023.
Medical records were analysed retrospectively. Data collected included demographic information, medical history, physical examination data, laboratory and instrumental examination data, treatment methods, and histological results.
The diagnosis of TSH-PA was made on the basis of hormonal examination (inadequate TSH level with high free T4 and/or free T3), clinical presentation, and pituitary magnetic resonance imaging (MRI) data. Some patients were treated with long-acting octreotide (20 mg intramuscularly once every 28 days or lanreotide 120 mg subcutaneously once every 28 days for at least two months).
TSH, free T4 and free T3 levels were measured before and after surgery using Architect i2000SR (Abbott Laboratories, Abbott Park, Illinois, USA), reference intervals: TSH (0.25–3.5 mIU/L), free T4 (9–20 pmol/L), free T3 (2.5–5.5 pmol/L).
All patients were tested for mixed PA secretion: blood tests were performed to determine the levels of anterior pituitary hormones and their target organs (GH, PRL, follicle-stimulating hormone (FSH), luteinising hormone (LH), insulin-like growth factor 1 (IGF-1), morning blood cortisol, estradiol, testosterone). In some patients, it was considered necessary to perform an oral glucose tolerance test with 75 g glucose and determine GH levels (at 0, 30, 60, 90, 120 minutes) to exclude/confirm acromegaly. Collagen type I C-telopeptide (CTx), osteocalcin, sex hormone binding globulin (SHBG) and total cholesterol levels were also measured to rule out tissue thyrotoxicosis.
Instrumental studies included MRI, thyroid ultrasound (to exclude thyroid enlargement/nodular mass), X-ray densitometry of the lumbar spine and proximal femur, electrocardiography and echocardiography.
Short-acting octreotide injections were given as part of the preoperative preparation to reduce thyroid hormone levels.
Surgical treatment of TSH-PA was performed in 36 patients, 34 of whom were operated on at the Endocrinology Research Centre. The surgical material was stained with hematoxylin and eosin according to the standard technique. In addition, in most cases, immunohistochemical (IHC) study of postoperative material was performed to confirm the diagnosis: antibodies against TSH, GH, PRL, Ki-67, adrenocorticotropic hormone (ACTH), FSH, LH, somatostatin subtype 2 receptors, dopamine subtype 2 receptors, and CAM5.2 were measured. If no IHC study was performed, the diagnosis of TSH-PA was confirmed by the achievement of postoperative remission.
Normalisation of TSH, free T4, and free T3 levels, regression of thyrotoxicosis symptoms, and absence of residual tumour tissue on MRI over time were considered criteria for postoperative remission and drug remission.
Ethical expertise
The study protocol was approved by Endocrinology Research Centre’s local ethical committee on 22 March 2023, session record no. 4.
Statistical analysis
The sample size was not calculated beforehand. Statistica 13.3 (StatSoft USA), SPSS 23 (Armonk, NY, IBM Corp) software was used for statistical processing of the data. The Shapiro-Wilk and Kolmogorov-Smirnov criteria were used to analyse sample distribution. Descriptive statistics data are presented as median and 25th and 75th percentiles. Absolute (n) and relative values (%) were calculated to describe qualitative data. The Mann-Whitney test was applied for intergroup comparisons on quantitative attributes. Pearson’s χ-square test and Fisher’s exact test were used to analyse relationships between categorical variables. The Spearman rank correlation method was used to assess relationship between traits. Logistic regression was performed to predict the probability of the event occurrence. Differences at p<0.05 were considered statistically significant. Bonferroni correction was applied to eliminate the effect of multiple comparisons.
RESULTS
Patient profile
Data from the Russian Hypothalamic-Pituitary Tumour Registry (HPTR) from 85 regions of the Russian Federation identified 11,754 patients with PA as of 29 March 2023, of whom 0.07% (n=8) had TSH-PA (excluding the cases presented in this article). Our single-centre study included 45 patients with TSH-PA. The median (Me) age was 45 years [ 30; 57]; the male to female ratio was 1:2.2 (14 males). The mean time to diagnosis of TSH-PA was three years [ 2; 6], with a maximum of 31 years.
The most common clinical manifestations of TSH-PA were: cardiac rhythm disorders (CRDs) – 82.2% (n=37) (sinus tachycardia was the most common CRD (in 30 cases)); neurological disorders (headache) – 53.3%; decreased bone mineral density (BMD) – in 46.7% of cases (osteoporosis – 22.2%; four patients had low traumatic fractures). Weight loss was observed in only 22.2% of cases (BMI 24.4 kg/m² [ 20.9; 28.3]). Menstrual dysfunction was observed in 23.8% of women of reproductive age (n=21). One patient had only neurological disorders due to a pituitary macroadenoma, and the TSH-PA diagnosis was made by IHC only. Thyroid pathology was detected in 27/45 patients (60%): in most cases nodular thyroid masses were detected (22.2%, n=10); Me of thyroid volume was 16 ml [ 11.4; 26.6]. All clinical manifestations of TSH-PA are listed in Table 1. One patient had undergone thyroid surgery prior to the diagnosis of TSH-PA; a subtotal thyroidectomy was performed after a misdiagnosis of diffuse toxic goiter; histological examination of the surgical material revealed a microfocus of papillary follicular carcinoma T1N0Mx. 26/45 patients (57.7%) had received thyrostatic medication in the past.
Table 1: TSH-PA clinical manifestations
|
Parameter |
Value |
|
Cardiac rhythm disorders, n (%) |
37/45 (82.2) |
|
sinus tachycardia |
30 (66.7) |
|
atrial fibrillation |
6 (13.4) |
|
ventricular premature beats |
1 (2.2) |
|
Thyroid pathology, n (%) |
27/45 (60) |
|
diffuse enlargement |
7 (15.6) |
|
nodular lesions |
10 (22.2) |
|
both of the above |
10 (22.2) |
|
Neurological disorders (headaches), n (%) |
24/45 (53.3) |
|
Low BMD |
21/45 |
|
osteoporosis |
10 (22.2) |
|
osteopenia |
11 (24.4) |
|
Tremor, n (%) |
16/45 (35.6) |
|
Menstrual disorders, n (%) |
5/21 (23.8) |
|
Weigh loss, n (%) |
10/45 (22.2) |
|
Eyesight disorders, n (%) |
4/45 (8.9) |
|
Family history of the disease, n (%) |
0/45 (0) |
Laboratory findings
At baseline, 21 patients had elevated TSH, free T3, and free T4; ten patients had elevated free T3 and free T4 with normal TSH; seven patients had elevated TSH and elevated free T3 or free T4; five patients had elevated one of the hormones (Figures 1–3). The median TSH was 4.41 mIU/L [ 3.02; 6.65], free T3 was 7.62 pmol/L [ 6.2; 10.5], free T4 was 22.6 pmol/L [ 19.48; 29.35]. It is also worth noting that TSH was slightly higher in patients receiving thyrostatic drugs than in those not taking them.
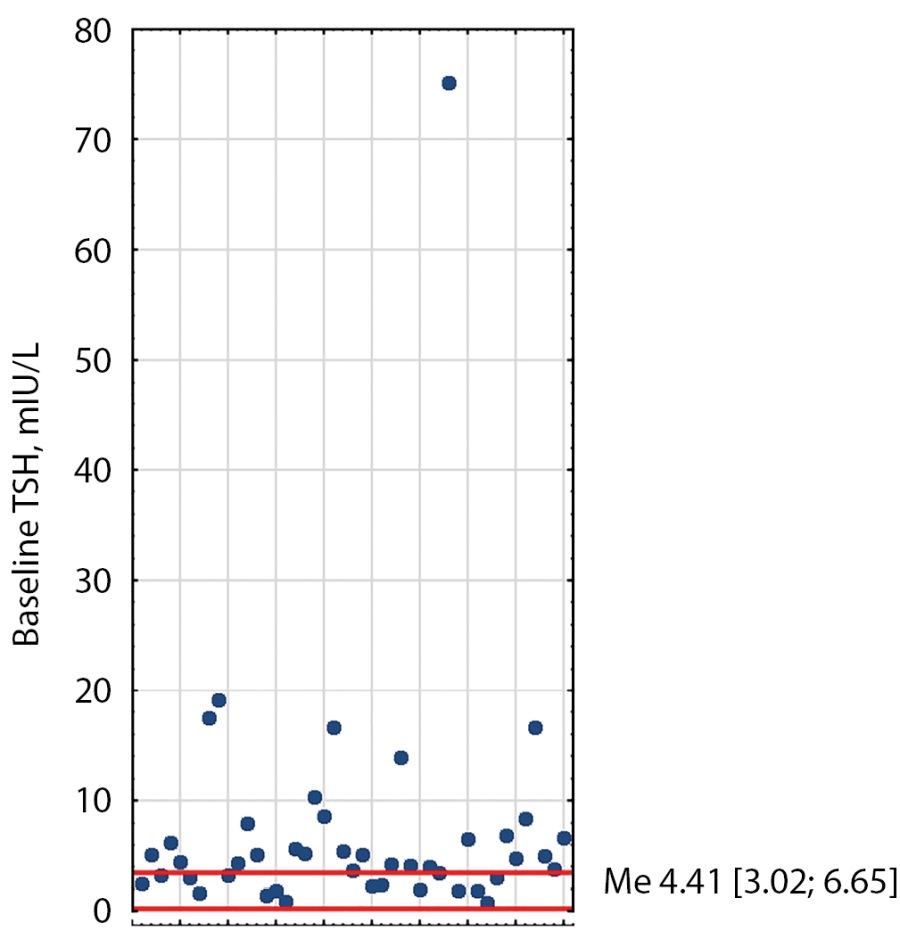
Figure 1: Distribution of baseline TSH values in TSH-PA patients. Red lines indicate reference intervals (0.25-3.5). Me [Q1; Q3] - median, interquartile range.
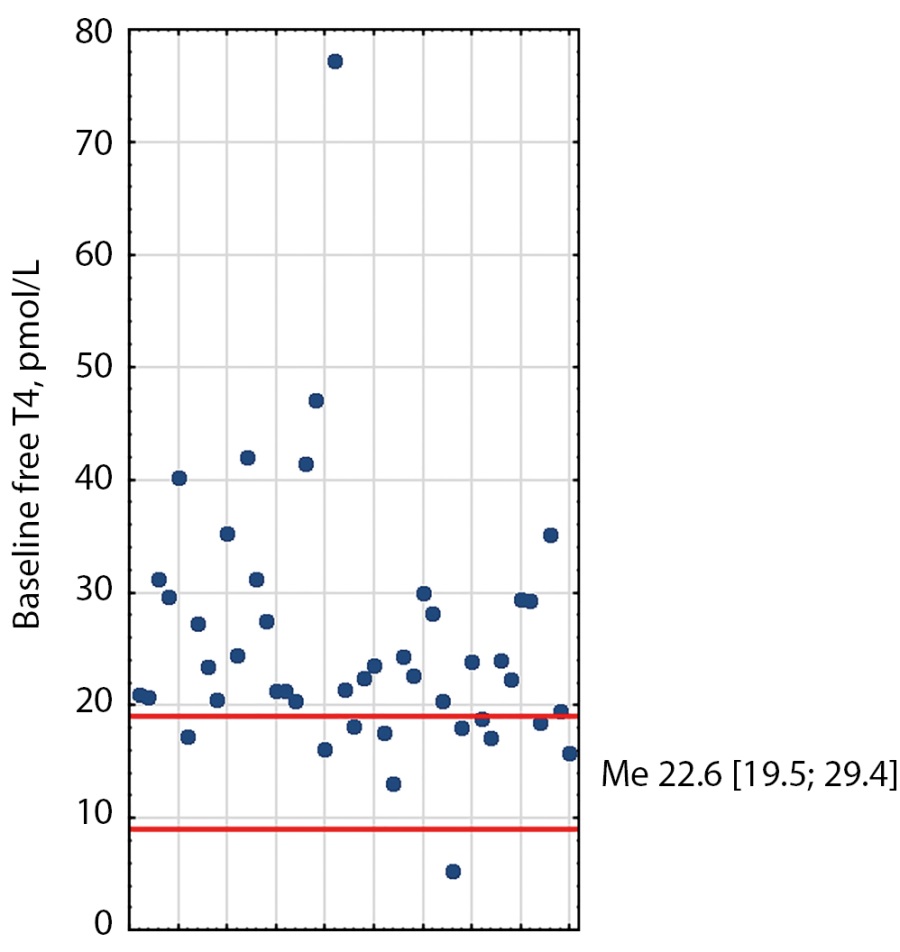
Figure 2: Distribution of baseline free T4 values in TSH-PA patients. Red lines indicate reference intervals (9-19). Me [Q1; Q3] - median, interquartile range.
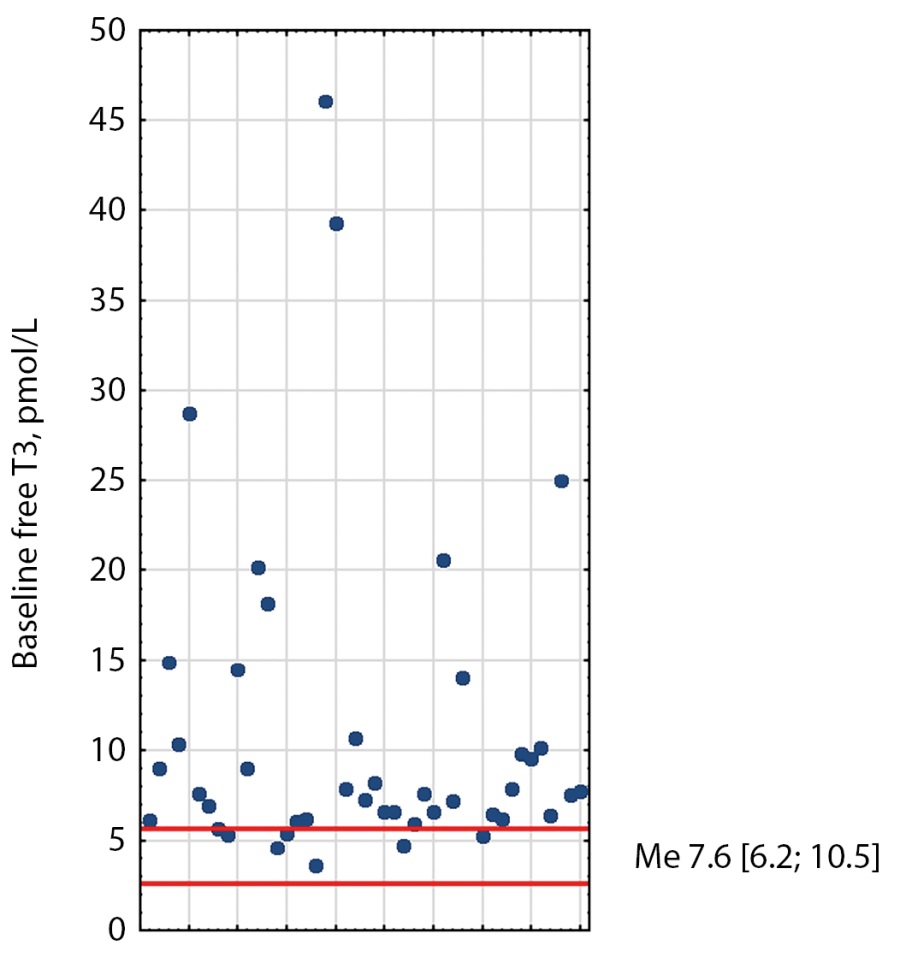
Figure 3: Distribution of baseline free T3 values in TSH-PA patients. Red lines indicate reference intervals (2.6–5.7). Me [Q1; Q3] - median, interquartile range.
Of the patients with elevated hormones
1) One patient was taking 5 mg of thiamazole on admission, free T3 and free T4 were normal, TSH was slightly elevated.
2) One patient with a history of subtotal thyroidectomy had an elevated TSH only.
3) One patient was found to have a combination of primary hypothyroidism and TSH-PA (signs of autoimmune thyroiditis on ultrasound; elevated TPOAb; normal free T3 and free T4 with elevated TSH and no significant suppression on supraphysiological doses of levothyroxine sodium in combination with PA).
4) Two patients were not taking any thyrostatic or other medication at the time of admission and each had an elevation of one of the hormones: free T4 or free T3.
One patient with no symptoms of thyrotoxicosis (only neurological symptoms) did not undergo free T3 testing, and her TSH and free T4 values were within the reference interval.
Elevated levels of SHBG, CTx and osteocalcin were found in 60.53%, 54.55% and 39.29% of cases respectively. The levels of total cholesterol, low-density lipoprotein, alkaline phosphatase and antibodies to the TSH receptor did not show clinically significant differences. Co-secretion of GH/TSH was detected in eight cases; hyperprolactinaemia due to mass effect was diagnosed in seven cases. No co-secretion of TSH/ACTH was found in our cohort of patients.
Correlation analysis showed no correlation between TSH levels and free T4/free T3 (p>0.05), nor were correlations found between TSH/free T4/free T3 levels and maximum PA size (p>0.05).
Test with somatostatin analogues
Somatostatin analogue test was performed in 39/45 patients. Short-acting octreotide test was performed in 26 patients; 15 of them had complete normalisation of thyroid hormones; in 6 patients, TSH and free T3 levels decreased to normal values and free T4 approached the upper limit of the normal interval.
Long-acting octreotide test was performed in 13/39 patients. Seven patients achieved complete normalisation of thyroid hormones; one patient showed normalisation of TSH levels, while free T4 and/or free T3 levels approached the upper limit of the normal interval. One patient with GH/TSH-PA normalised thyroid hormones, but not insulin-like growth factor-1. Octreotide 20 mg every 28 days was given to 11 patients and lanreotide 120 mg to two patients.
Side-effects of SA were observed in eight patients: diarrhoea in six cases, abdominal pain in five cases. Nausea, vomiting, flatulence, steatorrhoea, weight loss and gallstone formation were also observed within one year.
Magnetic resonance imaging
The majority of PAs were macroadenomas (77.8%) with a mean tumour diameter of 15.5 mm [ 12; 26] (see Table 2). Extrasellar spread of the tumour was noted in 21 cases.
Table 2: MRI findings in TSH-PA patients
|
PA feature, n (%) |
|
|
Macroadenoma |
35 (77.8) |
|
max tumour diameter (mm), Me [Q1; Q3] |
15.5 [ 12; 26] |
|
extracellular growth |
21 (60) |
|
Microadenoma |
10 (22.2) |
|
max tumour diameter (mm), Me [Q1; Q3] |
5 [ 4; 6] |
Treatment results
Prior to surgery, 28/36 patients received octreotide to achieve euthyroid status and reduce clinical symptoms: six received long-acting octreotide and 22 received short-acting octreotide. The mean duration of SA administration prior to surgery was one week [ 0.5; 2.2] for short-acting octreotide and 12 weeks [ 8; 29] for long-acting octreotide. Five patients received long-acting octreotide at a dose of 20 mg every 28 days, and one patient received 120 mg of lanreotide.
Surgery was performed in 36/45 patients (29 with macroPA and seven with microPA). After primary surgery, 31 patients (86.1%) achieved early postoperative remission. Median postoperative TSH was 0.161 mIU/L [ 0.016; 0.479]; median free T3 was 3.06 pmol/L [ 2.77; 3.73]; median free T4 was 14.21 pmol/L [ 10.75; 16.63]. Fifteen patients had TSH levels below 0.1 mIU/L, another 15 patients had levels between 0.1 and 1.0 mIU/L, and four patients had levels between 1.0 and 3.7 mIU/L.
Tumour invasion was detected during surgery in 12 cases (macroPA – ten cases; microPA – two cases). IHC analysis of resected material was performed in 24 patients. TSH expression was detected in 23 PA cases and isolated cells expressing TSH in one case. Somatostatin type 2 receptor (SSTR2) expression was detected in all 15 cases where this examination was performed, and D2R expression was detected in all ten cases where this examination was performed. Ki-67 IHC was performed on 18 PA specimens: 4 specimens had less than 1% Ki-67, three had between 1% and 3%, and four had more than 3% (Me=4 [ 1.8; 10.4]).
After surgery, no remission was diagnosed in five patients and three patients had a relapse (Table 3). Two patients underwent repeat surgery (one patient with no remission and one patient with relapse); post-surgery, both patients achieved remission.
Table 3: Summary of TSH-PA patients treatment results
|
Treatment method |
Number of patients |
||
|
n, (%) |
Remission |
Relapse/ no remission |
|
|
Surgical treatment |
36/45 (80) |
31 |
5/3 |
|
Repeat surgery |
2/8 (25) |
2 |
- |
|
Long-acting SA therapy prior to surgery |
6/36 (13.9) |
4 |
2/- |
|
Post-surgery radiotherapy |
2/36 (5.6) |
0 |
2/- |
|
Post-surgery long-acting SA therapy |
4/8 (50) |
4 |
- |
|
Long-acting SA therapy after radiotherapy |
2/2 (100) |
2 |
- |
|
Cabergoline |
9/45 (20) |
- |
9/- |
|
Long-acting SA therapy (unoperated patients) |
9/45 (20) |
3 |
6/- |
Two patients underwent postoperative radiotherapy, and in both cases remission was achieved only by subsequent administration of long-acting SA.
Long-acting SA therapy was prescribed in four patients after unsuccessful surgical treatment; remission was achieved in all cases with SA administration at doses of 10, 20, 30 mg once every 28 days; remission lasted for two years or more in three patients. Nine patients received dopamine receptor agonists, but euthyroidism was not achieved in any case of DRA monotherapy.
Hypopituitarism was noted in 4/34 patients (9.8%) preoperatively and in 10/34 (32.3%) postoperatively. All four patients had secondary hypogonadism before surgery and two had secondary adrenal insufficiency.
Postoperative complications developed in 11 (34.4%) patients: four patients had hypopituitarism; two patients had syndrome of inadequate ADH secretion; three patients had transient diabetes insipidus; one patient had transient diabetes insipidus and secondary adrenal insufficiency; one patient developed GH deficiency. One patient had both pre- and post-operative secondary adrenal insufficiency and secondary hypogonadism. Two patients are currently on levothyroxine sodium replacement therapy for secondary hypothyroidism.
The median follow-up after surgery was 12 months [ 1,5; 24]. Two patients with early postoperative remission were lost to follow-up. In a sample of patients with remission after at least 6 months of follow-up, a cut-off point for postoperative TSH (on the third day after surgery) was calculated using ROC analysis to identify patients at risk of recurrence. Those who did not achieve remission and those who subsequently relapsed had postoperative TSH levels above 0.391 mIU/L according to Youden’s criterion (AUC, 0.952; 95% CI 0.873–1.000; standard error 0.04; p<0.001), specificity (SP) = 88.9%, sensitivity (SE) = 100%.
Nine out of nine patients who did not undergo surgery were prescribed long-acting SAs. Surgery was not performed due to contraindications or patient refusal. Only 3/9 patients achieved remission at the end of follow-up with SA therapy (20 and 30 mg once every 28 days). During treatment with long-acting SA, PA size decreased in all patients, more so for macroPAs (initial size – 18×23×20 mm; size at last observation – 12×18×15 mm).
Of the non-surgical patients, a total of six had not achieved remission by the end of follow-up; of the surgical patients, 1/36 had not achieved remission (lost to follow-up).
The 4/6 patients who did not achieve remission were on long-acting SA therapy; to date, the SA dose has been increased to achieve disease control.
One unoperated patient with severe thyrotoxicosis and giant TSH-PA died of occlusive hydrocephalus before remission was achieved. Another death occurred in a patient in remission after reoperation – a mental disorder developed against the background of uncontrolled desmopressin administration for postoperative diabetes insipidus, which did not resolve after stabilisation of the thyroid status and normalisation of the sodium level; the destabilisation of the mental status led to stupor with pneumonia, which was the cause of death.
Preoperative factors affecting surgical treatment outcome
To determine preoperative factors affecting the outcome of surgical treatment, patients were divided into two groups: long-term remission (n=18) and relapse/no remission (n=6). The absence of laboratory thyrotoxicosis and residual PA tissue on MRI were considered remission criteria. Patients with early postoperative remission were not included in the analysis; the minimum follow-up period was 6 months and the maximum follow-up period was 3 years.
Comparative analysis of the remission and relapse/no remission groups showed no statistically significant differences in preoperative levels of TSH, free T3, free T4, visual impairment, maximum tumour size or tumour invasion. Despite the lack of differences in the above parameters, two characteristics were selected for logistic regression analysis to determine the preoperative factors influencing surgical treatment outcome: PA invasion at surgery and maximum PA size.
The analysis showed that each 1 mm increase in PA size increased the odds of relapse/no remission by a factor of 1.15 (OR 1.15, 95% CI 0.995–1.346, p=0.058); in the relapse/no remission group, the odds of PA invasion during surgery were 5.129 times higher than in the remission group (OR=5.129, 95% CI 0.284–92.648; p=0.268). Given the trend of the p-criterion, it is likely that an increase in the number of patients in both groups could lead to an increase in the power of the results.
DISCUSSION
Our paper presents the clinical characteristics and treatment outcomes of 45 TSH-PA patients. According to Russian HPTR data, rare TSH-PAs account for only 0.45% of the total number of PAs. In the international literature, TSH-PAs also account for 0.5% to 2% of all PAs [1], and the prevalence in the general population is 1–2 cases per million people, although the Swedish registry has recently reported an increase to 2.8 cases per million people [15]. The increase in the number of reported cases is most likely due to improved diagnostic methods (laboratory and instrumental) as well as physician awareness of the disease [22–24].
As in our study (n=35 (77.8%)), the detection rate of TSH-PA macroadenomas is still high worldwide; for example, in a systematic review by De Herdt C. et al. the majority of TSH-PAs (76.9%) were macroadenomas. However, evidence of a steady increase in microadenomas in recent years has been reported: in a study by Yamada S. et al, the number of microadenomas in newly diagnosed cases was significantly higher than in earlier years (p=0.0274) [16]; a meta-analysis by Cossu G. et al found that the percentage of microadenomas increased significantly in papers published after 2000 (p=0.04) [4].
In the literature, TSH-PA occurs with equal frequency in men and women, in contrast to other more common thyrotoxic diseases in which the female sex predominates [11]; the age at diagnosis can be any, but in most patients it is in the fifth to sixth decade of life and is approximately 42–46 years [1, 4, 5, 16]. In our study, the male-to-female ratio was 1:2.2, which differs slightly from data collected in international practice, but the mean age was 45 years, as in other studies [ 30; 57].
Approximately 1/3 of TSH-PA cases are misdiagnosed as Graves’ disease or functional autonomy, increasing the time to correct diagnosis and appropriate treatment [15]. Some patients may undergo unnecessary 131I radioiodine therapy (RIT) and thyroidectomy, which in turn may contribute to an increased incidence of invasive macroadenomas [1]. However, it should not be forgotten that TSH-PA can be associated with Graves’ disease [25], with primary hypothyroidism [26], and the role of long-term uncompensated primary hypothyroidism in the development of secondary hyperplasia/secondary TSH-PA has been discussed in the literature [27][28]. In our cohort of patients, the mean time to diagnosis of TSH-PA was three years [ 2; 6], with a maximum of 31 years; 26/45 patients (57.7%) were taking thyrostatic drugs, and one patient had undergone thyroid surgery (no PA growth after thyroidectomy, according to MRI). One patient in our study had a combination of primary hypothyroidism with TSH-PA (confirmed by ICH); after transnasal transsphenoidal adenomectomy, the patient has been in remission for three years and continues to receive hormone replacement therapy with levothyroxine sodium.
In patients with TSH-PA, signs and symptoms of thyrotoxicosis are usually milder than in patients with other causes of thyrotoxicosis or may be absent [1][5][9]. In addition, in mixed GH/TSH-PA, acromegaly symptoms may be more prominent, so it is important to assess all pituitary hormones in PA patients [1]. In our work, many patients had mild clinical manifestations of TSH-PA, and one patient had no signs of thyrotoxicosis; only symptoms of tumour mass effect (headaches) were observed. Cardiac arrhythmias were observed in 82.2% of patients, mainly sinus tachycardia (n=30); atrial fibrillation was diagnosed in six cases, which is consistent with data from other studies [5][9][29]. Most TSH-PAs are macroadenomas at the time of diagnosis, which explains the presence of mass effect symptoms in 30%-40% of patients [1][30]. In our study, neurological disorders (headaches) were reported in slightly more than half of the patients (n=24) and visual disturbances in four patients. Co-secretion of GH was detected in eight patients and prolactin elevation in seven patients, but we did not detect LH/FSH or ACTH expression in our cohort. Hypersecretion of GH and/or PRL is most common and is present in approximately 30% of TSH-PA patients, most likely due to shared transcription factors such as Prop-1 and Pit-1 [31]. Mixed TSH/FSH or LH-PA is much less common, and to date no TSH/ACTH-PA has been reported, probably due to the distant origin of the corticotrophic and thyrotrophic lineages [1].
Diffuse enlargement of the thyroid gland was found in 7/45 patients, nodular masses in 10/45, and a combination of the above in 10. The increased incidence of thyroid cancer in TSH-PA patients is controversial. For example, it has been suggested that the increased risk of thyroid cancer in TSH-PA patients may be due to the stimulatory effect of TSH hypersecretion on thyrocytes [32]; or that the higher prevalence of thyroid cancer in TSH-PA patients may be related to the frequent use of ultrasound in this particular cohort of patients [5]. In our case series, thyroid cancer was detected in one patient long before the correct diagnosis of TSH-PA was made.
The diagnosis of TSH-PA may be suggested when a patient has a high or insufficiently normal TSH level with elevated free T4 and/or free T3. The first step in diagnosing central hyperthyroidism is to repeat the thyroid status measurement to exclude the effect of various substances on the laboratory equipment itself [33] and to exclude transient changes in thyroid hormone levels due to certain conditions, such as medication (estrogens, amiodarone), pregnancy, acute psychiatric and critical conditions, subacute thyroiditis, or genetic causes, including resistance to thyroid hormones or familial dysalbuminemic hyperthyroxinemia. In addition, TSH-PA should be suspected in patients with primary hypothyroidism of various aetiologies in whom normalisation of the TSH level cannot be achieved by increasing the dose of levothyroxine sodium, provided the patient is compliant [29]. In addition to the above causes, thyroid hormone resistance (THR) syndrome should be differentiated from TSH-PA because of the similarity in clinical manifestations and laboratory parameters [34][35]. THR syndrome is a dominantly inherited disorder caused by mutations in the THRB gene (80%-85% of cases), with a prevalence of 1 case per 40,000 population. Patients with THR syndrome may be asymptomatic (most commonly) or have symptoms of thyrotoxicosis (less commonly), depending on the refractoriness of peripheral tissues to high levels of free T4 and free T3. TSH-PA can be distinguished from THR syndrome by: the presence of a first-degree relative with similar symptoms, normal α-subunit level, absence of PA mass effect symptoms, elevated TSH on thyroliberin test, no response to SAs administration, and evaluation of other peripheral blood parameters, particularly SHBG and CTx [1][5][9].
Han R. et al. performed a differential diagnosis of TSH-PA patients and THR syndrome patients using short-acting SAs [13]. In both TSH-PA and THR syndrome patients, the TSH level decreased at the beginning of the SA trial (after the first injection); however, the TSH suppression rate at 24 hours compared to 2 and 0 hours was significantly higher in TSH-PA patients than in THR syndrome patients (70.58±18.6% vs. 6.01±25.41%, p<0.0001, 79.83±12.79% vs. 51.16±13.62%, p<0.0001, respectively). In our study, short-acting octreotide was administered to 26/39 patients, of whom 15 achieved complete normalisation of thyroid hormones, and long-acting octreotide was tested in 13/39 patients, of whom 7 achieved complete normalisation of thyroid hormones. Side effects occurred in 20.5% of patients, mainly in the gastrointestinal tract. In general, in the differential diagnosis of TSH-PA and THR syndrome, a test with long-acting SAs administered for at least two months can be used with confidence, whereas a test with short-acting SAs may have low sensitivity and is currently only used for the management of thyrotoxicosis in preparation for surgery.
Examination of TSH levels alone when thyroid hormone dysfunction is suspected may also complicate the diagnosis of TSH-PA and lead to inappropriate management of patients, as TSH levels are often normal with elevated free T4 and/or free T3 [16]. In our study, 16/45 patients (35.6%) had normal TSH levels at the time of diagnosis.
As mentioned above, diagnostic tests that measure α-subunit levels can be used to diagnose TSH-PA. In patients with TSH-PA, elevated α-subunit levels can be observed in 70% of cases and are most common in macroadenomas; therefore, a normal α-subunit level makes the diagnosis of TSH-PA less likely, but does not exclude it [9]. In the thyrotropin-releasing hormone test (200 μg thyroliberin intravenous injection), relatively healthy controls and patients with THR syndrome show an increase in TSH levels, in contrast to TSH-PA patients in whom no change in TSH levels is observed in 85% of cases. The triiodothyronine test is performed by administering 80–100 μg of triiodothyronine per day for eight to ten days, followed by TSH measurement; in TSH-PA, in contrast to other conditions, no suppression of TSH levels occurs in 100% of cases. However, this test is contraindicated in the elderly and those with cardiovascular disease because it can cause arrhythmias and is not available in many countries [1][9][6]. The long-acting SA test can also be recommended for the diagnosis of TSH-PA. For example, most TSH-PA patients have a significant decrease or normalisation of TSH, free T4 and/or free T3 levels after SA injection. The use of SAs as neoadjuvant therapy prior to surgery contributes to the reduction of PA; however, some studies have found no statistically significant differences in the improvement of surgical outcomes between patients with and without octreotide administration [4][16][22][36]. In the Russian Federation, tests with thyrotropin-releasing hormone and triiodothyronine, α-subunit measurement are not performed; clinical practitioners use tests with short- or long-acting SAs. In our study, short- and long-acting SAs were tested in 39/45 patients, of whom 22 showed complete hormone normalisation; 28/36 patients continued to receive SAs preoperatively; the Me of duration of administration of short-acting SAs was one week and long-acting SAs 12 weeks. SAs were not used in microadenomas without significant thyroid hormone elevation and marked symptoms of thyrotoxicosis.
Pituitary MRI is recommended only after laboratory confirmation of TSH-PA-induced central thyrotoxicosis. Imaging studies can not only confirm the presence of TSH-PA, but also help to define the location of the tumour and its relationship to the surrounding tissue. Most TSH-PAs are tumours larger than 10 mm [9][22]; however, as mentioned above, micro-TSH-PAs have recently been diagnosed with increasing frequency. If the MRI does not show a tumour in the sella turcica, but the laboratory tests indicate a TSH-PA, one should look for TSH ectopia [37]. We found that MRI data showed more macroadenomas with a maximum tumour diameter of 15.3 mm [ 12; 27], whereas microadenomas had a maximum diameter of 5 mm [ 4; 6]. As in other studies [5][16], we observed no correlations between TSH/free T4/free T3 levels and PA size.
The first-line treatment for TSH-PA patients is transnasal transsphenoidal adenomectomy followed by IHC of postoperative material. Studies suggest that approximately 80% of TSH-PA patients can achieve remission with surgical treatment alone [5][9]. Compared to other PAs, TSH-PA tends to have a higher degree of microinvasion and intra- and peri-tumoural fibrosis, which may negatively affect surgical outcome and cause perioperative complications such as bleeding, liquorrhoea, diabetes insipidus and hypopituitarism [9][22]. In their study of a large group of TSH-PA patients, Yamada S. et al. first identified preoperative factors that may influence surgical outcome. The most significant predictors of non-radical surgery in patients were the degree of cavernous sinus invasion and the maximum PA diameter – the larger the tumour diameter and the more pronounced the degree of invasion, the more often no remission was observed after surgery. Neither TSH and free T4 levels, nor age or gender influenced the outcome [16].
In our study, the remission rate after primary surgery was relatively high, as it is internationally: 86.1% (31/36 patients). Tumour invasion during surgery was detected in 12 patients, mostly with macro-PA. IHC was performed in 24 patients and showed strong expression of THS in 23 cases; in one PA case, only isolated THS-expressing cells were detected. In the study by Yamada S. et al [16], when isolated THS-expressing cells were found in the tumour, proteinase K treatment was performed to confirm the diagnosis; in our study, this treatment was not performed and the diagnosis was confirmed by achieving biochemical remission of the underlying disease. After performing logistic regression analysis to diagnose preoperative factors affecting disease remission, we found that each 1-mm increase in PA size increased the odds of relapse/no remission by a factor of 1.15, which is consistent with the results of previous studies [4][5][16][20]. However, we did not obtain statistically significant results for the parameter ‘invasion during surgery’, but given the trend of the p-criterion, it is possible to obtain significant results in a larger group of patients. Parameters such as TSH, free T4, free T3, IGF-1, visual impairment, sex or age did not influence the outcome of surgery.
Due to the rarity of the disease and the variety of methods used to diagnose remission, no clear criteria have been established for the cure of TSH-PA patients. Criteria for complete TSH-PA remission include: absence of clinical picture of thyrotoxicosis and neurological symptoms; absence of tumour visible on imaging studies; and normalisation of serum TSH, free T4, and free T3 within 3–6 months after surgery [9]. In contrast to somatotropin, the exact postoperative TSH threshold for determining remission has not been established. It is known that an undetectable TSH level (less than 0.01 mU/L) can be a good indicator of radical TSH-PA removal [4][7][19]. Some authors also establish remission by the response to thyrotropin-releasing hormone stimulation or triiodothyronine test, which is the most sensitive and specific test to confirm complete PA removal [19][29]. Therefore, there is no single standard for postoperative remission criteria, and the above factors should be considered together.
Kim SH et al. evaluated postoperative TSH levels (2, 6, 12, 18, 24 hours postoperatively) to identify patients at risk of disease recurrence in the early postoperative period. The authors found that a TSH greater than 0.62 μME/mL at 12 hours was the best predictor of relapse risk [20]. We also calculated the cut-off point of postoperative TSH (taken three days after surgery) in our cohort to identify patients at risk of relapse. Our analysis showed that patients without long-term remission had TSH levels higher than 0.391 mIU/L in the first week after surgery (SP=88.9%, SE=100%), which may further help clinicians in determining patient management strategies and individual follow-up timeframes.
Radiotherapy and/or drug therapy with SAs are alternative treatments for TSH-PA. When surgery is not possible/successful or refused by the patient, they should be considered.
Most thyrotropic cells express varying amounts of SSTRs, especially SSTR 2 and SSTR 5. Published data indicate that SAs are effective in about 90–95% of cases and contribute to tumour shrinkage in 30–50% of patients [1][30]. SAs are relatively safe despite their side effects (prevalence 10–30%) such as cholelithiasis, impaired carbohydrate metabolism and diarrhoea. Dopamine receptors are also present in thyrotropic cells, so dopamine receptor agonists can also be used in some TSH-PA cases, but with low efficacy; positive effects are mainly observed in patients with mixed PRL/TSH-PA secretion [38].
The efficacy of radiotherapy is about 37% [39]. However, due to the increasing availability of SAs and the risk of hypopituitarism, this type of therapy is now used much less frequently. Nevertheless, radiotherapy is relatively more common in poorly differentiated Pit-1 lineage tumours and is also suitable for patients with contraindications to surgery and drugs and for patients with residual tumour tissue [22].
In our study, four patients with relapse/no remission after primary surgery were treated with long-acting SAs and all achieved remission, which was attributed to SSTR2 expression by IHC. Of the nine unoperated patients, three achieved remission with long-acting SAs and a 30% reduction in PA size was observed. Only two patients underwent radiotherapy, and in both cases remission was achieved only after administration of SAs. Neither monotherapy with dopamine receptor agonists nor the addition of dopamine receptor agonists to existing therapy led to disease stabilisation. This suggests that the prescription of long-acting SAs may be effective in the combined treatment of TSH-PA patients.
The schema for diagnosis and management of TSH-PA patients may therefore be as follows (Figure 4):
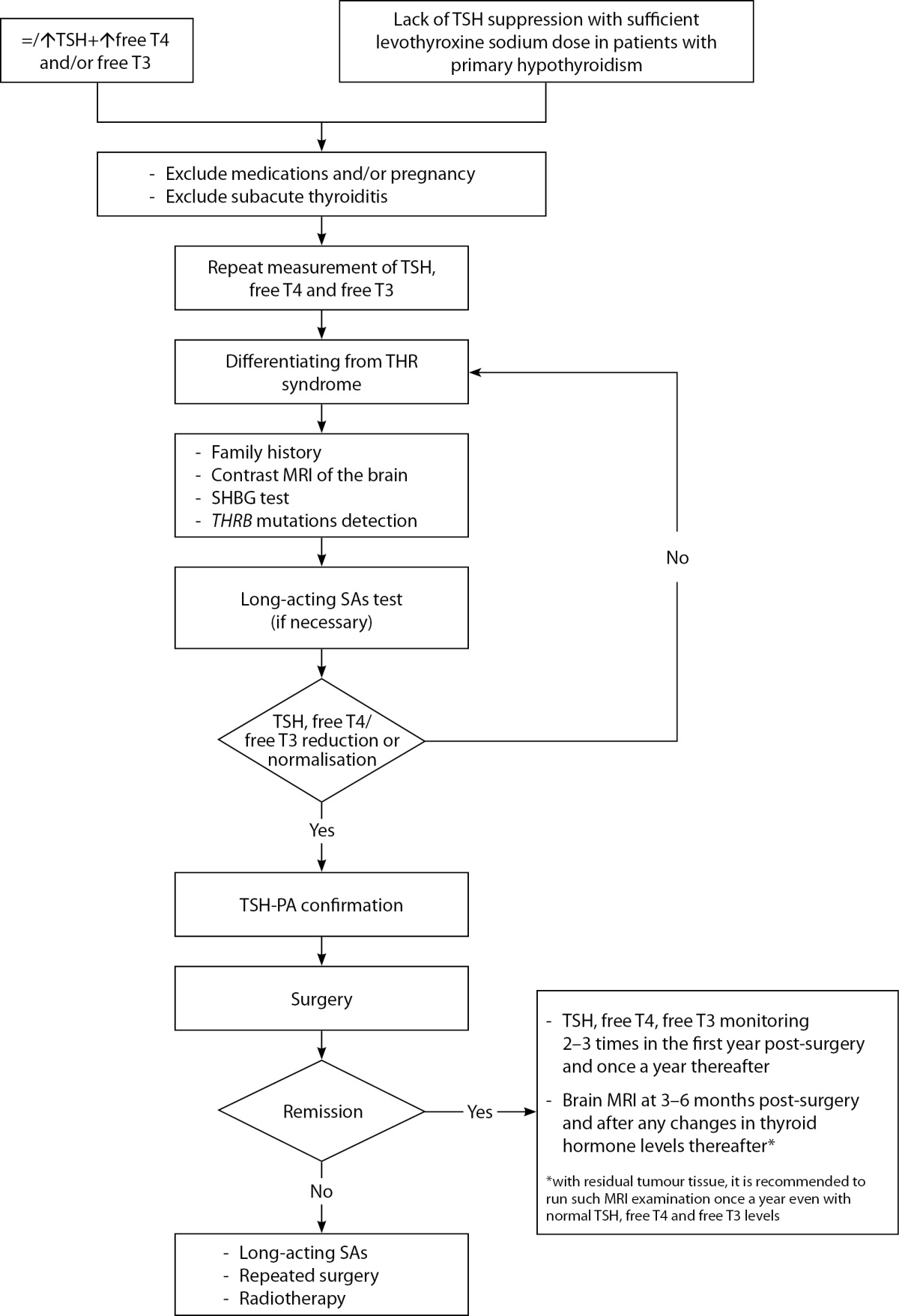
Figure 4: The scheme of diagnostics and management of TSH-PA patients
Abbreviations: TSH – thyroid stimulating hormone; free T4 – thyroxine; free T3 – triiodothyronine; THR syndrome – thyroid hormone resistance syndrome; THRB – Thyroid hormone receptor beta; MRI – Magnetic resonance imaging; SHBG – Sex hormone binding globulin; SAs – Somatostatin analogues.
Limitations of this study
Due to the retrospective study design, there are some limitations to the analysis, including the lack of structured and consistent follow-up of patients, changes in treatment methods over time, incomplete data on certain parameters (e.g. IHC), and loss of patients to follow-up; the unavailability of some TSH-PA diagnostic methods is another limitation.
CONCLUSION
TSH-PAs are an extremely rare type of PA and a relatively uncommon cause of thyrotoxicosis; as a result, most remain undetected until the macroadenoma stage. Early diagnosis and differential diagnosis of these tumours is extremely important because of the frequent mistreatment of TSH-PA patients. Unfortunately, none of the methods available in Russia (thyroid hormone levels, brain MRI, SHBG measurement, CTx measurement, octreotide test) is ideal for confirming the diagnosis of TSH-PA, so they must be used in combination. The most effective treatment is transnasal transsphenoidal adenomectomy (achieving remission in 86.1% of cases). If surgery is ineffective, contraindicated, or the patient refuses surgery, SA can be used as second-line therapy to control the disease (100% remission with combination therapy and 42.9% with monotherapy). Large PA size and invasion at surgery may generally be associated with an unfavourable outcome of surgical treatment. We also proposed a possible model of postoperative TSH level (>0.391 mU/L) for predicting TSH-PA recurrence; the model needs to be validated in a larger group of patients.
ADDITIONAL INFORMATION
Source of funding. This study was funded by a grant from Russia’s Ministry of Education and Science, contract no. 075-15-2022-310 dated 20 May 2022.
Conflict of interest. The authors hereby declare they have had no apparent or potential conflict of interest related to this study and the publication thereof.
Authors’ contribution. All authors confirm that their authorship fulfils the ICMJE international criteria (all authors have made substantial contributions to the conception, research and preparation of the article; all authors read and approved the final draft prior to publication).
References
1. Beck-Peccoz P, Persani L, Lania A. Thyrotropin-Secreting Pituitary Adenomas. In: Feingold KR, Anawalt B, Blackman MR, et al., eds. Endotext. South Dartmouth (MA): MDText.com, Inc.; October 13, 2022
2. Lee W, Cheung AS, Freilich R. TSH-secreting pituitary carcinoma with intrathecal drop metastases. Clin Endocrinol (Oxf ). 2012;76(4):604-606. doi: https://doi.org/10.1111/j.1365-2265.2011.04288.x
3. Jailer JW, Holub DA. Remission of Graves’ disease following radiotherapy of a pituitary neoplasm. Am J Med. 1960;28:497-500. doi: https://doi.org/10.1016/0002-9343(60)90181-9
4. Cossu G, Daniel RT, Pierzchala K, et al. Thyrotropin-secreting pituitary adenomas: a systematic review and meta-analysis of postoperative outcomes and management. Pituitary. 2019;22(1):79-88. doi: https://doi.org/10.1007/s11102-018-0921-3
5. De Herdt C, Philipse E, De Block C. ENDOCRINE TUMOURS: Thyrotropin-secreting pituitary adenoma: a structured review of 535 adult cases. Eur J Endocrinol. 2021;185(2):R65-R74. Published 2021 Jul 12. doi: https://doi.org/10.1530/EJE-21-0162
6. Rebrova DV, Sleptsov IV, Chernikov RA, et al. TSH secreting pituitary tumor — an experience of 20 years follow-up. Clinical and experimental thyroidology. 2020;16(2):31-41. (In Russ.). doi: https://doi.org/10.14341/ket12430
7. Astafeva LI, Kadashev BA, SHishkina LV, et al. Clinical and morphological characteristics, diagnostic criteria, and outcomes of surgical treatment of TSH-secreting pituitary adenomas. Zhurnal Voprosy Neirokhirurgii. 2016;80(6):24-35. (In Russ.). doi: https://doi.org/10.17116/neiro201680624-35
8. Klimchuk AV, Yatskov IA, Bubley KV, Enzel DA, Sherbakov AS. TSH-рroducing pituitary microadenoma: diagnostic problems in the debut of the disease. Problems of Endocrinology. 2022;68(3):44-49. (In Russ.). doi: https://doi.org/10.14341/probl12860.
9. Beck-Peccoz P, Giavoli C, Lania A. A 2019 update on TSH-secreting pituitary adenomas. J Endocrinol Invest. 2019;42(12):1401-1406. doi: https://doi.org/10.1007/s40618-019-01066-x
10. Korbonits M, Kumar AV. AIP Familial Isolated Pituitary Adenomas. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews®. Seattle (WA): University of Washington, Seattle; June 21, 2012
11. Vanderpump MPJ. (2019). Epidemiology of Thyroid Disorders. In: Luster M, Duntas L, Wartofsky L. (eds) The Thyroid and Its Diseases. Springer, Cham. doi: https://doi.org/10.1007/978-3-319-72102-6_6
12. Gatto F, Grasso LF, Nazzari E, et al. Clinical outcome and evidence of high rate post-surgical anterior hypopituitarism in a cohort of TSH-secreting adenoma patients: Might somatostatin analogs have a role as first-line therapy? Pituitary. 2015;18(5):583-591. doi: https://doi.org/10.1007/s11102-014-0611-8
13. Han R, Shen L, Zhang J, et al. Diagnosing Thyrotropin-Secreting Pituitary Adenomas by Short-Term Somatostatin Analogue Test. Thyroid. 2020;30(9):1236-1244. doi: https://doi.org/10.1089/thy.2019.0470
14. Beck-Peccoz P, Persani L, Mannavola D, Campi I. Pituitary tumours: TSH-secreting adenomas. Best Pract Res Clin Endocrinol Metab. 2009;23(5):597-606. doi: https://doi.org/10.1016/j.beem.2009.05.006
15. Ónnestam L, Berinder K, Burman P, et al. National incidence and prevalence of TSH-secreting pituitary adenomas in Sweden. J Clin Endocrinol Metab. 2013;98(2):626-635. doi: https://doi.org/10.1210/jc.2012-3362
16. Yamada S, Fukuhara N, Horiguchi K, et al. Clinicopathological characteristics and therapeutic outcomes in thyrotropin-secreting pituitary adenomas: a single-center study of 90 cases. J Neurosurg. 2014;121(6):1462-1473. doi: https://doi.org/10.3171/2014.7.JNS1471
17. Beck-Peccoz P, Lania A, Persani L. TSH-producing adenomas: in Endocrinology, Adult and Pediatric (7th Edition, vol. I), edited by Jameson LJ and DeGroot LJ. Sauderns Elsevier, Philadelphia, PA, 2015 pp.266-274
18. van Varsseveld NC, Bisschop PH, Biermasz NR, et al. A long-term follow-up study of eighteen patients with thyrotrophin-secreting pituitary adenomas. Clin Endocrinol (Oxf ). 2014;80(3):395-402. doi: https://doi.org/10.1111/cen.12290
19. Beck-Peccoz P, Lania A, Beckers A, et al. 2013 European thyroid association guidelines for the diagnosis and treatment of thyrotropin-secreting pituitary tumors. Eur Thyroid J. 2013;2(2):76-82. doi: https://doi.org/10.1159/000351007
20. Kim SH, Ku CR, Na M, et al. Immediate postoperative measurement of thyroid-stimulating hormone as an early predictor of remission in thyroid-stimulating hormone-secreting pituitary adenomas. J Neurosurg. 2020;134(3):794-800. Published 2020 Mar 13. doi: https://doi.org/10.3171/2020.1.JNS192787
21. Amlashi FG, Tritos NA. Thyrotropin-secreting pituitary adenomas: epidemiology, diagnosis, and management. Endocrine. 2016;52(3):427-440. doi: https://doi.org/10.1007/s12020-016-0863-3
22. Luo P, Zhang L, Yang L, An Z, Tan H. Progress in the Pathogenesis, Diagnosis, and Treatment of TSH-Secreting Pituitary Neuroendocrine Tumor. Front Endocrinol (Lausanne). 2020;11:580264. Published 2020 Nov 27. doi: https://doi.org/10.3389/fendo.2020.580264
23. Petrik G.G., Kosmacheva E.D., Polyakova U.I., et al. TSH-secreting pituitary adenoma: late diagnosis and effectiveness of therapy. Problems of Endocrinology. 2017;63(1):39-45. (In Russ.) doi: https://doi.org/10.14341/probl201763139-45]
24. Tkachuk A.V., Grebennikova T.A., Lapshina A.M., et al. TSH-secreting pituitary adenoma in combination with primary hypothyroidism in the outcome of Hashimoto’s disease: diagnostic difficulties. Clinical and experimental thyroidology. 2018;14(3):162-168. (In Russ.) doi: https://doi.org/10.14341/ket10021
25. Fu J, Wu A, Wang X, Guan H. Concurrent Graves’ Disease and TSH Secreting Pituitary Adenoma Presenting Suppressed Thyrotropin Levels: A Case Report and Review of the Literature. Front Endocrinol (Lausanne). 2020;11:523. doi: https://doi.org/10.3389/fendo.2020.00523
26. Myers A, Hatanpaa KJ, Madden C, Lingvay I. Thyrotropin-secreting adenoma in a patient with primary hypothyroidism. Endocr Pract. 2011;17(6):e135-e139. doi: https://doi.org/10.4158/EP11127.CR
27. Franceschi R, Rozzanigo U, Failo R, et al. Pituitary hyperplasia secondary to acquired hypothyroidism: case report. Ital J Pediatr. 2011;37:15. doi: https://doi.org/10.1186/1824-7288-37-15
28. Du J, Ji H, Jin J, Gao S, Yan X, Hu S. Pituitary adenoma secondary to primary hypothyroidism: Two case reports. Medicine (Baltimore). 2020;99(8):e19222. doi: https://doi.org/10.1097/MD.0000000000019222
29. Amlashi FG, Tritos NA. Thyrotropin-secreting pituitary adenomas: epidemiology, diagnosis, and management. Endocrine. 2016;52(3):427-440. doi: https://doi.org/10.1007/s12020-016-0863-3
30. Tjörnstrand A, Nyström HF. DIAGNOSIS OF ENDOCRINE DISEASE: Diagnostic approach to TSH-producing pituitary adenoma. Eur J Endocrinol. 2017;177(4):R183-R197. doi: https://doi.org/10.1530/EJE-16-1029
31. Pereira BD, Raimundo L, Mete O, et al. Monomorphous Plurihormonal Pituitary Adenoma of Pit-1 Lineage in a Giant Adolescent with Central Hyperthyroidism. Endocr Pathol. 2016;27(1):25-33. doi: https://doi.org/10.1007/s12022-015-9395-2
32. Perticone F, Pigliaru F, Mariotti S, et al. Is the incidence of differentiated thyroid cancer increased in patients with thyrotropin-secreting adenomas? Report of three cases from a large consecutive series. Thyroid. 2015;25(4):417-424. doi: https://doi.org/10.1089/thy.2014.0222
33. Kim EI, Dimitrova DА, Dimitrova DA, et al. Endogenous and exogenous interferences in thyroid function immunoassays. Clinical and experimental thyroidology. 2020;16(3):16-24. (In Russ.). doi: https://doi.org/10.14341/ket12698
34. Moran C, Chatterjee K. Resistance to thyroid hormone due to defective thyroid receptor alpha. Best Pract Res Clin Endocrinol Metab. 2015;29(4):647-657. doi: https://doi.org/10.1016/j.beem.2015.07.007
35. Gurnell M, Visser TJ, Beck-Peccoz P, Chatterjee VK. Resistance to Thyroid Hormone. In: Jameson LJ, DeGroot LJ, editors. Endocrinology, Adult and Pediatric (7th Edition, vol. II). Philadelphia, PA: Sauderns Elsevier; 2016. Pp. 1648-1665. doi: 10.1016/b978-0-323-18907-1.00095-0.
36. Fukuhara N, Horiguchi K, Nishioka H, et al. Short-term preoperative octreotide treatment for TSH-secreting pituitary adenoma. Endocr J. 2015;62(1):21-27. doi: https://doi.org/10.1507/endocrj.EJ14-0118
37. Ortiz E, Peldoza M, Monnier E, et al. Ectopic pituitary adenoma of the TSH-secreting sphenoidal sinus with excellent response to somatostatin analogs. Theory of the embryogenesis and literature review from a clinical case. Steroids. 2020;154:108535. doi: https://doi.org/10.1016/j.steroids.2019.108535
38. Kao YH, Chang TJ, Huang TS. Thyrotropin-secreting pituitary tumor presenting with congestive heart failure and good response to dopaminergic agonist cabergoline. J Formos Med Assoc. 2013;112(11):721-724. doi: https://doi.org/10.1016/j.jfma.2012.07.015
39. Malchiodi E, Profka E, Ferrante E, et al. Thyrotropin-secreting pituitary adenomas: outcome of pituitary surgery and irradiation. J Clin Endocrinol Metab. 2014;99(6):2069-2076. doi: https://doi.org/10.1210/jc.2013-4376
About the Authors
D. A. TrukhinaRussian Federation
Diana A. Trukhina, MD
11 Dm. Ulyanova street, 117036 Moscow
Competing Interests:
Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с проведенным исследованием и публикацией настоящей статьи.
E. G. Przhiyalkovskaya
Russian Federation
Elena G. Przhiyalkovskaya, MD, PhD
Moscow
Competing Interests:
Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с проведенным исследованием и публикацией настоящей статьи.
Zh. E. Belaya
Russian Federation
Zhanna E. Belaya, MD, PhD, Professor
Moscow
Competing Interests:
Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с проведенным исследованием и публикацией настоящей статьи.
A. Yu. Grigoriev
Russian Federation
Andrey Yu. Grigoriev, PhD, MD, Professor
Moscow
Scopus ID: 57190411198
Competing Interests:
Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с проведенным исследованием и публикацией настоящей статьи.
V. N. Azizyan
Russian Federation
Vilen N. Azizyan, PhD, MD
Moscow
Competing Interests:
Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с проведенным исследованием и публикацией настоящей статьи.
E. O. Mamedova
Russian Federation
Elizaveta O. Mamedova, MD, PhD
Moscow
Competing Interests:
Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с проведенным исследованием и публикацией настоящей статьи.
L. Ya. Rozhinskaya
Russian Federation
Liudmila Ya. Rozhinskaya, MD, PhD, Professor
Moscow
Competing Interests:
Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с проведенным исследованием и публикацией настоящей статьи.
A. M. Lapshina
Russian Federation
Anastasia M. Lapshina, MD, PhD
Moscow
Competing Interests:
Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с проведенным исследованием и публикацией настоящей статьи.
E. A. Pigarova
Russian Federation
Ekaterina A. Pigarova, MD, PhD
Moscow
Competing Interests:
Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с проведенным исследованием и публикацией настоящей статьи.
L. K. Dzeranova
Russian Federation
Larisa K. Dzeranova, MD, PhD
Moscow
Competing Interests:
Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с проведенным исследованием и публикацией настоящей статьи.
N. M. Platonova
Russian Federation
Nadezhda M. Platonova, MD, PhD
Moscow
Competing Interests:
Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с проведенным исследованием и публикацией настоящей статьи.
E. A. Troshina
Russian Federation
Ekaterina A. Troshina, MD, PhD, Professor
Moscow
Competing Interests:
Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с проведенным исследованием и публикацией настоящей статьи.
G. A. Melnichenko
Russian Federation
Galina A. Melnichenko, MD, PhD, Professor, acad.
Moscow
Competing Interests:
Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с проведенным исследованием и публикацией настоящей статьи.
Supplementary files
|
|
1. Figure 1. Distribution of baseline TSH values in patients with TSH-AH. Red lines indicate reference intervals (0.25–3.5). Me [Q1; Q3] — median, interquartile range. | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(111KB)
|
Indexing metadata ▾ | |
|
|
2. Figure 2 Distribution of baseline fT4 values in patients with TSH-AH. Red lines indicate reference intervals (9–19). Me [Q1; Q3]—median, interquartile range. | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(116KB)
|
Indexing metadata ▾ | |
|
|
3. Figure 3. Distribution of baseline fT3 values in patients with TSH-AH. Red lines indicate reference intervals (2.6–5.7). Me[Q1; Q3]—median, interquartile range. | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(122KB)
|
Indexing metadata ▾ | |
|
|
4. Figure 4. Diagnostic and management scheme for patients with TSH-AH. | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(552KB)
|
Indexing metadata ▾ | |
Review
For citations:
Trukhina D.A., Przhiyalkovskaya E.G., Belaya Zh.E., Grigoriev A.Yu., Azizyan V.N., Mamedova E.O., Rozhinskaya L.Ya., Lapshina A.M., Pigarova E.A., Dzeranova L.K., Platonova N.M., Troshina E.A., Melnichenko G.A. Thyrotropin-secreting pituitary adenomas: clinical features and results of treatment in 45 patients. Problems of Endocrinology. 2024;70(2):23-36. https://doi.org/10.14341/probl13325

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0).









































.jpg)


