Scroll to:
IGG4-related diseases in endocrinology
https://doi.org/10.14341/probl12285
Abstract
Immunoglobulin-G4-related disease (IgG4-RD) is a chronic immunomediated pathology of different organs of local or systemic nature, which has been established as a separate clinical entity in the early 2000s and is characterized by storiform fibroid inflammation of the affected tissues, their increase, and elevated serum immunoglobulin-G4 (IgG4) levels. The most common manifestations of the disease are major salivary and lacrimal gland enlargement, lymphadenopathy and type 1 autoimmune pancreatitis (AIP1), however, other organs may be also involved (the thyroid, eyes, meninges, heart, lungs, kidneys, aorta, upper airways, mesentery, etc.).
The effectiveness of treatment of IgG4-RD, as well as other pathological conditions, is also determined by the timely diagnosis. However, the latter is complicated due to the variety of clinical manifestations and rather variable diagnostic criteria. It is necessary to constantly update the evidence-based knowledge and diagnostic algorithms within this pathology in order to overcome the difficulties, and involve immunologists, endocrinologists, pathologists and specialists in other spheres.
This review provides information about the etiology, pathogenesis, and current methods of diagnosis and treatment of IgG4-related diseases, as well as examples of some manifestations of IgG4-RD that an endocrinologist may face in practice.
Keywords
For citations:
Rumyantsev P.O., Kozlov I.G., Kolpakova E.A., Chukhacheva O.S., Korenev S.V., Goncharov A.G., Ulanova E.U. IGG4-related diseases in endocrinology. Problems of Endocrinology. 2020;66(2):24-32. https://doi.org/10.14341/probl12285
CLASSIFICATION AND FUNCTIONS OF VARIOUS IMMUNOGLOBULIN CLASSES
Immunoglobulins are one of the most important factors of humoral immunity and provide protection against all types of pathogens and most tumor cells. In humans, immunoglobulins are represented by 5 classes (G, M, A, E and D). At the initial contact of immunocompetent cells with foreign antigens, the synthesis of specific class M immunoglobulins (IgM) is initiated, then, under the influence of T cells and cytokines, B cells switch to the synthesis of class G immunoglobulins (IgG) and other classes. IgG make up 75% of the total amount of antibodies and are subdivided into 4 subclasses (IgG1-IgG4) depending on the presence of one of the four types of heavy chains (γ1-γ4) and the serum level. Quantitatively, most antibodies belong to the IgG1 subclass, the smallest amount is IgG4, constituting only 4% of the relative concentration of serum immunoglobulins [1].
Under normal conditions, the humoral immune response to most antigens results in the synthesis of antibodies from all four IgG subclasses. This makes it difficult to understand the role of each of the subclasses in the elimination of antigen from the body. According to one of the most probable concepts, it is assumed that the antibody response is extended in time with the gradual activation of all new classes and subclasses of immunoglobulins as soon as it is impossible to effectively eliminate the antigen by the previous ones. According to this concept, the primary (acute) response to an antigen begins with IgM (it has been repeatedly confirmed in experiment and clinical practice), then IgG3 (and / or IgE) is connected to it. If the elimination of antigen does not occur, the synthesis of IgG1 and IgG2 is activated. And finally, if this is not enough, the production of IgG4 begins. It is believed that in parallel with the switching of IgG subclasses, an increase in the affinity of antibodies occurs, due to which antibodies of the next subclass have an advantage in antigen binding, regardless of their total amount.
Thus, IgG4 is a marker of the chronicity of the immune response or an indicator of the multiple immune response to the same antigen. For example, an increase in the titers of IgG 4-antibodies is found in chronic parasitic infections, during long-term allergen-specific therapy in patients with atopy [2] or in chronic viral infections [3].
The exact function of IgG4 antibodies is unknown. From the proven facts: IgG4 antibodies are produced mainly in response to polysaccharide or carbohydrate antigens; are often bivalent and are not able to activate complement (which in total makes it difficult to eliminate the antigen from the body with their help); penetrate the placental barrier (the same as IgG1 and IgG3); have moderate affinity for Fc receptors on phagocytes, and mainly for the subtype of these receptors, which provides anti-inflammatory activity — obviously, from a clinical point of view, this should manifest itself in the form of a decrease in the symptomatology of diseases against the background of the continuation of the pathological process. This phenomenon is due to the production of anti-inflammatory cytokines when IgG4 combines with Fc receptors on phagocytes, which will be described in more detail below [4].
IGG4-АЗ
Many human diseases are accompanied by changes in serum and tissue levels of antibodies. For a long time, autoimmune pathological conditions with a similar histological picture, such as autoimmune pancreatitis, Küttner’s tumor, Riedel’s thyroiditis, sclerosing cholangitis, retroperitoneal fibrosis (including multifocal fibrosclerosis) and others, orbititis, mastitis, mastitis, otitis media, laryngitis, pharyngitis, rhinitis, rhinosinusitis, lymphadenitis, pancreatitis, cholangitis, pneumonia, pericarditis, mastitis, tubulointerstitial nephritis, prostatitis, aneurysms, etc.) were considered independent diseases (Fig. 1).

Figure 1. Variants of clinical manifestation of IgG4-associated diseases.
Only in recent decades have researchers from centers around the world started talking about a similar pathological mechanism for the development of these conditions and the possible syndromic manifestation of the same disease, which can fundamentally change approaches to their diagnosis and treatment. Some diseases, which were previously associated with the involvement of one organ, are now considered as manifestations of IgG4-associated diseases (IgG4-A3) [5].
IgG4-A3 were isolated into a separate group of autoimmune pathology only at the beginning of the XXI century, when in 2001 Hamano et al. revealed the relationship of all the diseases listed above with an increased level of IgG4 production. IgG4-A3 is a chronic immune-mediated pathology of various organs, characterized by moiré-like fibrous inflammation of the affected tissues, their increase (edema) and abundant infiltration of IgG4-positive plasma cells, as well as an increase in the level of serum IgG4 [6].
IgG4-A3 is often diagnosed at the stage of noticeable changes in the organ, dysfunction, or accidentally during a biopsy in an attempt to verify another pathology. If untreated, the disease leads to fibrosis and irreversible organ damage [7]. Most often, IgG4-A3 manifests itself as lesions of the salivary and lacrimal glands, lymphadenopathy, and type 1 autoimmune pancreatitis [8].
ETHIOLOGY OF IGG4-АЗ
As with other autoimmune diseases, the etiology of IgG4-A3 remains not fully understood. Traditionally, it is assumed that there is a genetic predisposition and the influence of a number of epigenetic factors in the initiation and further development of the disease.
Today, on the basis of genetic studies, some changes in the genome have been established that can create a statistical probability of a predisposition to IgG4-A3 [9]. These are, first of all, certain variations of genes related to the major histocompatibility complex (HLA-DR), and mononucleotide polymorphisms of significant genes (SNP). However, further research is required to verify the genetic components of the disease [9].
Due to the low practical significance of determining the genetic predisposition to incurable diseases in the absence of routine genome editing technologies, it is of much greater interest to establish epigenetic changes that lead to the onset and progression of the disease, in particular, to assess the pathogenic triggers non-IgG4-A3 (see next section).
IMMUNOPATHOGENESIS OF IGG4-A3
The exact immunopathogenesis of IgG4-A3 is currently unknown, which may be due to insufficient knowledge about the function of IgG4 in normal conditions. Unambiguously, in these diseases, there are violations of the T-helper (Th) balance and, in particular, changes in the Th2 regulating humoral response. But the question remains: is IgG4 a «witness» (marker) or pathogenic factor IgG4-A3.
Despite this, based on the properties of IgG4 and its role in the immune response to various antigens, it is possible to formulate a fairly reliable hypothesis of the development of IgG4-A3. As already mentioned, a number of characteristics of IgG4 make antibodies of this subclass «special» from the point of view of the implementation of the humoral immune response. The high affinity to the antigen compared to other IgG subclasses makes IgG4 a priority in the fight for antigen binding, but divalent (a practical analogue of monovalence) does not allow the formation of full-fledged immune complexes for antigen elimination, which contributes to its persistence. Low ability to bind and activate complement components is another mechanism of «tightening» and chronization of the immune response without the final elimination of the cause that causes it, i.e. antigen. And finally, the predominant interaction of IgG4 with inhibitory Fc receptors of phagocytes (FcyRIIB), through which the production of anti-inflammatory cytokines is triggered, creates a unique situation when a pathological process takes place without vivid symptoms, which usually forces the patient to see a doctor. This leads, on the one hand, to a late diagnosis of a local disease, and on the other, to its systematization and spread to many organs and tissues. Here are some facts supporting this hypothesis (Fig. 2) [10].
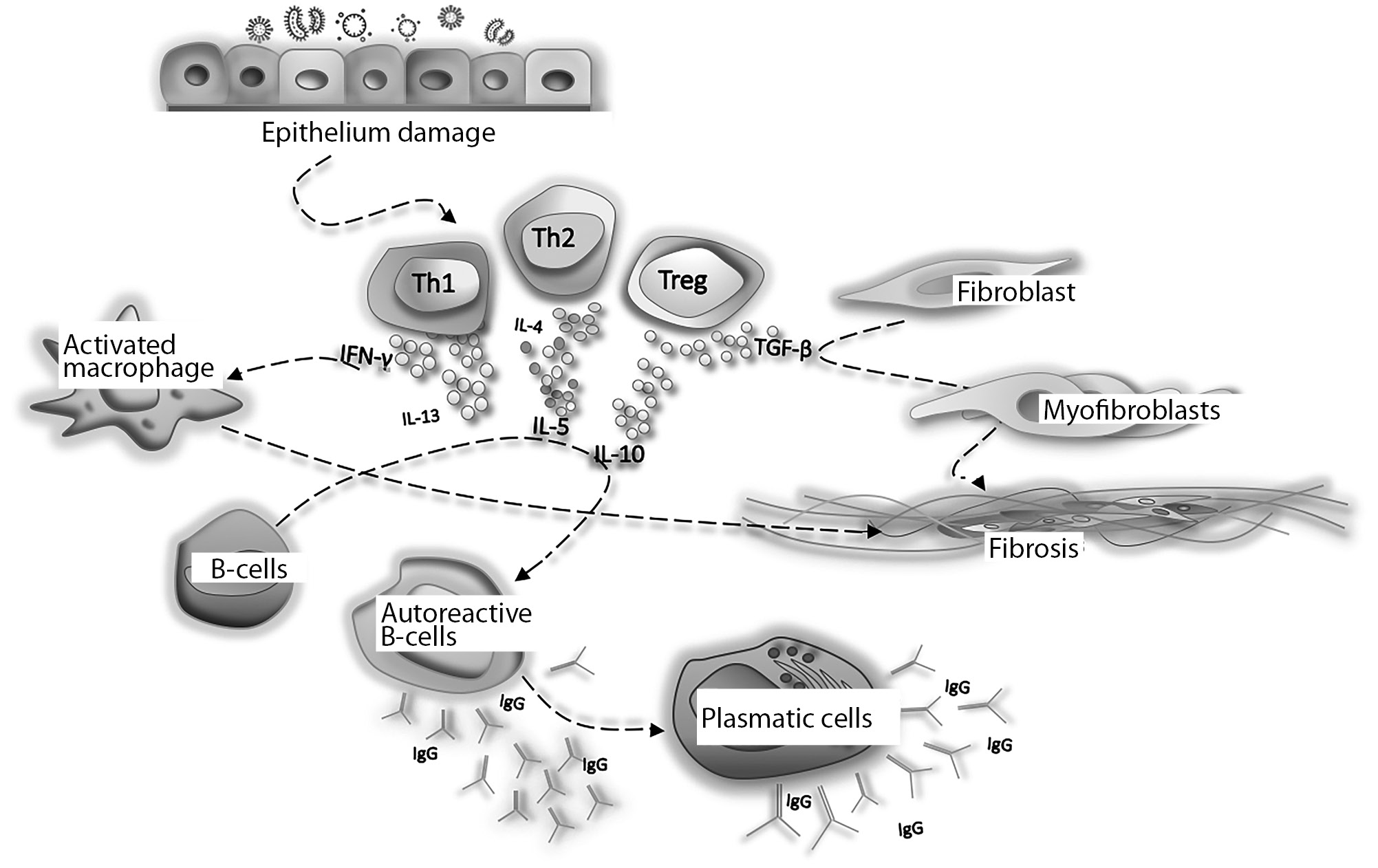
Figure 2. Scheme of immunopathogenesis of IgG4-associated diseases.
An abnormal immune response to tissue damage, allergens, commensal or pathogenic microorganisms with an imbalance in the Th2 response is obviously a trigger for the development of IgG4-A3. Simultaneously activated Th1, Th2 and Tregs produce IFN-y, IL-4, IL-5, IL-13, IL-10, IL-21 and TGF-0. Tregs produce TGF0-1, which activates fibroblasts and induces differentiation of endothelial and epithelial cells into myofibroblasts, resulting in tissue fibrosis [8]. IL-4 and IL-10 control the activation of autoreactive B cells to form IgG4 and IgE, and induce differentiation and replication of plasma cells into IgG4 +. IL-5, IL-13 and TGF-0 activate fibroblasts and eosinophils. IFN-y can also promote macrophage activation and fibrosis of damaged tissues; IgG4 and IgE antibodies can cross-react with an autoantigen. Activated autoreactive T cells can promote germinal center formation and attract more and more autoreactive B cells. IFN-y — interferon gamma; IL — interleukin; TGF-0 — transforming growth factor-beta; Th — T-helper; Treg — T regulatory cells
CLINICAL PICTURE
About 1/3 of patients have a history of atopic disease (bronchial asthma, allergic rhinitis, nasal polyps, atopic dermatitis). In the absence of a history of atopy, some patients may have eosinophilia and / or an increase in serum IgE levels [5, 7, 8]. Clinical symptoms of IgG4-A3 are presented quite widely, more often they manifest in one or more organs, simultaneously or sequentially. Diseases can be subacute or chronic, manifest themselves with a spectrum of mild local symptoms or massive tissue damage and organ failure. Among the most characteristic symptoms of manifestation are kidney dysfunction, general weakness, enlargement of the submandibular or parotid salivary glands, hearing loss, and thirst. This characteristic of IgG4-A3 suggests that the symptoms and features of the anamnesis are very, very variable, and this, in turn, imposes requirements on a certain level of specificity of diagnostic methods.
In 2019, US researchers identified the main four phenotypic groups of IgG4-A3 [11]. These groups suggest a structured structure that can be used to clarify the etiology of the disease, identify risk factors, and develop personalized treatment strategies. The presence of phenotypic IgG4-A3 groups contributes to early diagnosis, timely prescription of pathogenetic therapy and, as a consequence, minimization of irreversible organ damage [11]. Given the multiple organ nature of IgG4-A3, the potential number of phenotypic groups is on the order of several thousand, but most of them are not applicable for clinical practice or scientific research. The four groups listed below differ from each other in the distribution of affected organs, the patient’s demographic characteristics (race, sex, age), and serum IgG4 concentration.
- Group 1: damage mainly to the pancreato-hepatobiliary system. The probability of damage to the pancreas in this group, according to the study, was 87%, the gallbladder — 55%, the liver — 13%.
- Group 2: retroperitoneal fibrosis (53%) and/or aortitis (22%).
- Group 3: limited lesion of the structures of the head and neck according to the type of incomplete Mikulicz syndrome. The likelihood of damage to the lacrimal glands in this group was 60%; submandibular glands — 50%, parotid glands — 22%; orbits — 22%.
- Group 4: diseases of the head and neck according to the scheme corresponding to Mikulicz syndrome, as well as systemic multiple organ damage. The probability of damage to the parotid gland in group 4 was 49%; pancreas — 46%; lungs — 39%; kidneys — 36%; orbital damage probability <1%.
As for the age and sex characteristics of these groups, the proportion of female patients was significantly higher in group 3 (76%) compared to groups 1, 2 and 4, where this indicator was much lower and amounted to 21%, 26% and 22%, respectively [11 ]. The average age at diagnosis was lower in group 3 (55 years) compared to the other three groups (58 years — in group 2 and 63 years — in groups 1 and 4) [11]. The racial distribution also varied across groups. The proportion of Asian patients in groups 3 (67%) and 4 (52%) was significantly higher than in groups 1 (37%) and 2 (25%) [11]. Patients in group 4 had the highest serum IgG4 concentrations (median 1170 (interquartile range (IQR) 520–2178) mg / dl), followed by group 3 (445 (183–888) mg / dl) and group 1 (316 (147–622) mg / dl); group 2 had the lowest serum IgG4 concentrations (178 (63–322) mg / dL) [11].
DIAGNOSTICS
In 2011, Umehara et al. proposed criteria for the diagnosis of IgG4-A3 for practical use [12] (Fig. 3). However, due to the nonspecificity of symptoms and changes in the estimated parameters, the diagnosis of IgG4-A3 should be based on a comprehensive analysis of clinical symptoms, laboratory signs, results of histological examination and data of imaging methods.
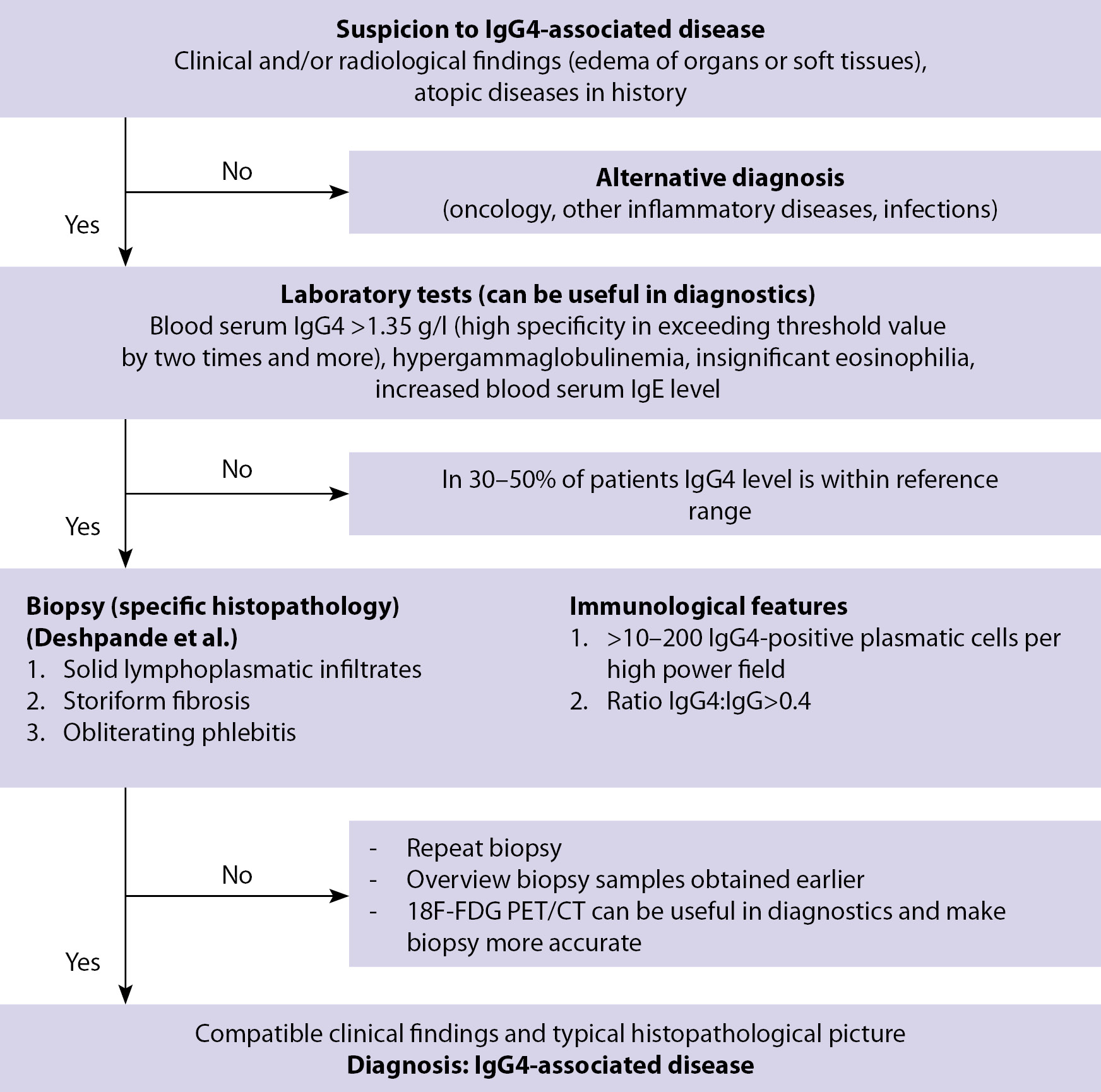
Figure 3. Algorithm for the diagnosis of IgG4-associated diseases (Umehara, et al.) [11]
In laboratory examination of patients with IgG4-A3, an increase in the level of IgG4 is usually detected, but its concentration cannot be used as the only diagnostic criterion due to its low specificity. Currently, the possibility of determining plasmablasts as a marker of the disease is being discussed, and the gold standard in the diagnosis of Ig4-AZ is a biopsy followed by a description of the histopathological picture [13].
In 2019, with the joint efforts of specialists from the American College of Rheumatology (ACR) and the European League against Rheumatism (EULAR), IgG4-AZ classification criteria were developed and tested with the highest possible specificity while maintaining a moderately high sensitivity [14]. The classification criteria ACR and EULAR are based on the assessment of serological blood tests, clinical presentation, histological examination of the affected organs and the characteristics of the topical diagnosis based on the results of radiation diagnostics [14]. The value of the developed set of criteria lies in the possibility of making a diagnosis of IgG4-AZ on the basis of the study, even without performing a biopsy of the affected organs while achieving a sufficient number of points [14]. A preliminary assessment of 20 points or more is defined as the starting point at which a clinical case meets the criteria for IgG4-AZ staging (specificity 97.8%, sensitivity 82.0%) [14].
Laboratory tests and cell markers in the diagnosis of IgG4-A3
Due to the heterogeneity of studies aimed at studying IgG4-AZ, it is difficult to determine the list of necessary parameters and their threshold values in the diagnosis of this pathology. The main biomarkers characteristic of IgG4-AZ are shown in Fig. 4. The most interesting are serological parameters, as well as fibrosis markers. According to various sources, the threshold value of IgG4 in the range of 1.35–1.44 g / l in blood serum has a sensitivity of 87.2–97.0% and a specificity of 79.6–82.6% in the diagnosis of IgG4- AZ [15, 16]. In one of the studies, it was shown that an increase in the threshold value of IgG4 to 2.8 g / L increases the specificity of the study to 96.2% and reduces the sensitivity to 56.9% [17]. Also informative is the ratio of IgG 4-positive plasma cells and IgG-positive plasma cells. Serum and tissue levels of IgG4 and IgG2 have diagnostic potential [18]. Soluble serum IL-2 receptor (sIL-2R) may potentially become a marker reflecting disease activity and response to treatment, but the available data are limited by a small sample. Therefore, further prospective studies are required to confirm the sensitivity and specificity of sIL-2R in the diagnosis of IgG4-AZ [18].
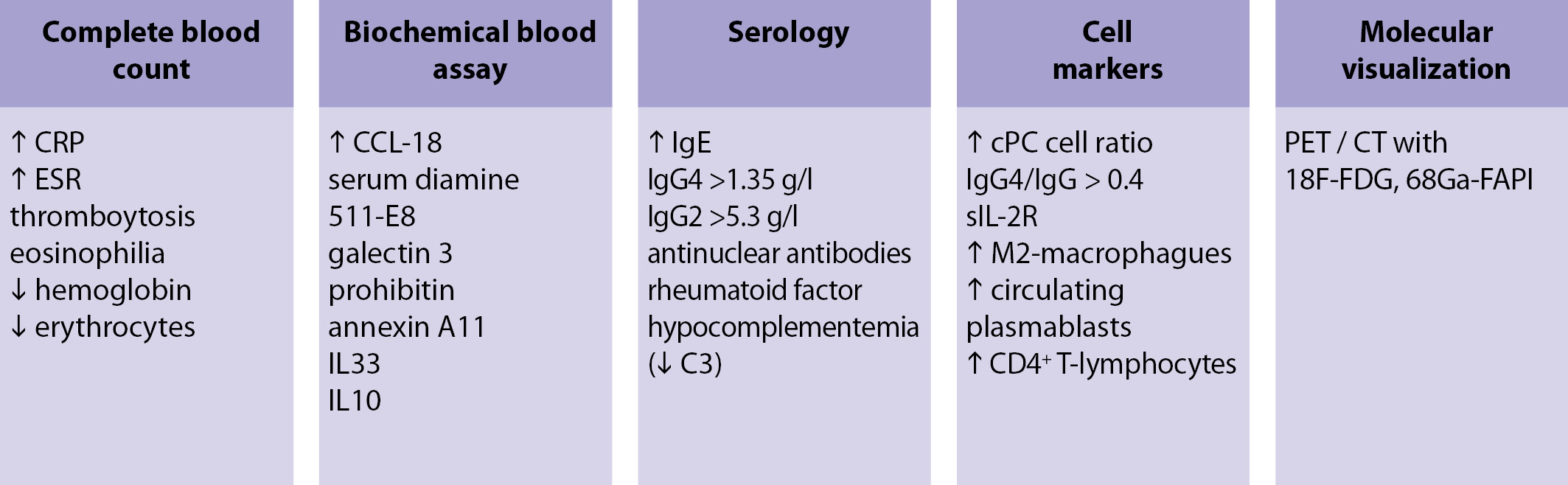
Figure 4. Suspected biomarkers IgG4-AZ
Since the main component of IgG4-AZ is fibrous inflammation, it is logical to assume that fibrosis markers can help in the diagnosis of this pathology. According to some reports, the serum level of the ligand CC-chemokine 18 (CCL18) was significantly higher in patients with IgG4-AZ than in the control group, and also directly correlated with the number of affected organs, disease activity, sIL2R level [19], tissue infiltration M2 -macrophages and the degree of fibrosis [20]. In addition, there is evidence of an increase in the serum level of laminin 511-E8 in patients with IgG4-AZ compared with the control group [21], and galectin-3, prohibitin and annexin A11 can take part in the pathophysiological processes in IgG4-AZ [19].
A cellular marker of inflammatory fibrosis can be М2-macrophages, therefore, cytokines associated with them (IL-33, IL-10, CCL18) [19]. Due to pronounced fibroinflammatory changes, an increase in the level of circulating plasmablasts can be considered as one of the markers of this group of diseases (sensitivity 95%, specificity 82%) [22, 23] and a predictor of the prognosis of therapy success [24]. CD4 + cytotoxic T-lymphocytes correlate with the activity of the disease and with the number of lesions [24].
Biopsy
Currently, biopsy data are the gold standard for IgG4-AZ diagnostics [5]. Diagnosis of IgG4-AZ is based on the histopathological picture in the organs involved, which must meet the following criteria:
1.the presence of solid lymphoplasmic infiltrates;
2.storiform fibrosis;
3.obliterating phlebitis;
4.eosinophilic infiltration [5, 25].
An important stage in the study of a biopsy is an immunohistochemical study, during which the ratio of IgG4 + / IgG + and the number of cells IgG4 + / proteins promoting hibernation (hpf) is determined [14].
Molecular imaging in the diagnosis of IgG4-A3
The prospects for the diagnosis of IgG4-A3 from the point of view of molecular imaging are very interesting. At first, it consisted in the use of WF-FDG PET / CT (fluorodeoxyglucose labeled with a radioactive isotope of fluorine-18, positron emission (PET) and computed tomography (CT)), which bore fruit: Allowed to determine the localization of areas of inflammation, to assess the spread of the disease, to make a decision on a biopsy and was used to monitor the response to therapy.
Recently, however, there have been reports of more informative imaging studies in the framework of IgG4-A3, namely 68Ga-FAPI PET / CT (inhibitor of fibroblast activator protein, labeled with the gallium-68 radioisotope PET / CT) [26, 27].
In August 2019, the experience of imaging IgG4-A3 using WF-FDG PET / CT and 68Ga-FAPI PET / CT was published. In a patient with a serum IgG4 titer of 11,300 mg / l (with a reference range of 80–1400 mg / l), according to WF-FDG PET / CT, areas of FDG accumulation in the lymph nodes of the neck and mediastinum and in the right lung were revealed; also areas with increased FDG accumulation were identified in the parotid and submandibular glands. According to 68Ga-FAPI PET / CT, an intensive accumulation of the radiopharmaceutical was revealed in the same areas as according to WF-FDG PET / CT, but in addition, areas of accumulation in the pancreas were found [28]. This makes 68Ga-FAPI more specific for the imaging of IgG4-A3 foci, which is probably due to the severity of the fibrous component [28].
IGG4-AZ IN THE PRACTICE OF THE ENDOCRINOLOGIST
IgG4-associated dacryoadenitis and sialoadenitis
Currently, Mikulicz disease (BM) and chronic sclerosing sialoadenitis (Kuttner tumor) are defined as part of the IgG4-A3 spectrum. Mikulicz disease is characterized by bilateral symmetric damage to the lacrimal and all groups of salivary glands due to massive lymphoplasmacytic infiltration without impairing their function. Mostly middle-aged women are ill. Unlike other diseases included in IgG4-A3, fibrosis is never formed in BM [29].
Kuttner tumor is a IgG4-associated sialoadenitis and affects one or both of the submandibular glands [30]. It is characterized by progressive periductal fibrosis, formation of lymphoid follicles, dilatation of ducts with massive lymphoplasmacytic infiltration, and acini atrophy [29].
Thyroid pathology (Riedel’ thyroiditis, fibrosing autoimmune thyroiditis)
Riedel thyroiditis (TR) is an extremely rare, slowly progressive inflammatory disease of the thyroid gland, in which one or both lobes are enlarged and replaced by connective tissue, which is accompanied by compression of adjacent neck structures; fibrosis often extends beyond the thyroid capsule and affects adjacent organs [7]. Usually the goiter is hard, the so-called «stony density», and is soldered to the surrounding tissues. Patients are worried about shortness of breath, hoarseness, difficulty breathing and swallowing [8].
Most patients with TR are characterized by euthyroidism. Thyroid dysfunction occurs due to extensive replacement of glandular tissue with non-functioning connective tissue [7]. In this case, hypothyroidism with increased titers of antibodies to thyroglobulin (AT TG) and thyroperoxidase (AT TPO) is most often detected. A review of 185 patients with TR from the Cleveland Clinic found that 64% had euthyroidism, 32% had hypothyroidism, and 4% had thyrotoxicosis [7].
In addition to TR, other pathologies of the thyroid gland are considered in the IgG4-A3 spectrum, namely fibrosing autoimmune thyroiditis (Hashimoto’s thyroiditis, AIT) and Graves’ disease. It is more common in young men with significantly increased levels of AT TG and AT TPO. According to the data of ultrasound examination, a reduced echogenicity of the thyroid tissue is characteristic [6]. Patients with N4-mediated AIT have a higher risk of developing hypothyroidism and a significant increase in the thyroid gland, which often serves as an indication for thyroidectomy [5].
IgG4-associated ophthalmopathy (IgG4-AО)
IgG4-AO tends to develop painless exophthalmos and diplopia. It is characterized by a recurrent course, and most often a bilateral enlargement of the lacrimal glands combined with IgG4-AO is demonstrated. Both IgG4-AO and non-IgG4-related idiopathic orbital inflammatory diseases usually respond to glucocorticoid therapy. Nevertheless, the frequency of relapses after cessation of glucocorticoids is usually higher in IgG4-AO (67% versus 30%), and relapse occurs earlier (the average duration of relapse is 1 month versus 5 months) [31].
IgG4-A3 THERAPY TODAY AND IN PROSPECT
According to the 2015 international consensus on the treatment of IgG-AZ, 87% of experts are of the opinion that drug therapy is indicated for all patients with symptoms of the active phase of the disease, while asymptomatic patients do not always require immediate initiation of therapy [32]. Nevertheless, the timely initiation of treatment leads to a faster and more complete remission. Glucocorticosteroids (GCS) or their combination with basic anti-inflammatory drugs (DMARDs) are proposed as first-line drugs for inducing remission [30, 32, 33].
In a 2019 meta-analysis, various options for induction of remission in patients with IgG4-AZ were considered [34]. Thus, the combination of corticosteroids and DMARDs is associated with a higher probability of remission compared to monotherapy with corticosteroids, DMARDs, and rituximab [34]. As a maintenance therapy, rituximab is most effective, and DMARD monotherapy is the safest in terms of the development of side effects [34].
Often, tamoxifen, a selective estrogen antagonist, is prescribed in combination with corticosteroids, which can be used as monotherapy (in patients with corticosteroids resistance) or as adjuvant therapy to reduce the corticosteroids dose. This drug reduces the expression of TGF-β, which can lead to a slowdown in tissue fibrosis. Several cases of reduction of the focus of connective tissue and reduction of symptoms of the underlying disease during therapy with tamoxifen have been described [7]. Clinical reviews discuss tamoxifen doses ranging from 10 to 20 mg / day [8]. In case of resistance to therapy with GCS / combination of GCS with tamoxifen or monotherapy with tamoxifen, treatment with rituximab is recommended [7]. Rituximab is genetically engineered chimeric mouse or human monoclonal antibodies with specificity for the CD20 antigen (CD20-AG) localized on the surface of pre-B-lymphocytes and mature B-lymphocytes. The structure of rituximab is IgG1. The Fab-fragment of rituximab binds to CD20-AG on lymphocytes and, with the participation of the Fc-domain, initiates immunological reactions mediating lysis of B cells [6]. A decrease in IgG4 levels against the background of insignificant changes in other IgG subclasses during rituximab therapy may be explained by the fact that IgG4 + cells are shorter-lived than cells expressing other IgG subclasses [35]. The antifibrosing effect of rituximab may be due to a decrease in myofibroblast activity in patients with IgG4-A3 [35]. Rituximab appears to be a promising drug for the treatment of IgG4-A3, especially in cases resistant to other therapy and with a history of relapse. At the same time, the basal levels of IgG4 and IgE in the blood serum are reliable predictors of recurrence [34].
Evaluation of the production of interferon type 1 (I-IFN) and inter-leukin-33 (I-IFN / IL-33) may be of interest as a therapeutic target in patients with IgG4-A3, since in these diseases the production of plasma cells increases. dendritic cells of I-IFN and, as a consequence, increases the level of IL-33, which plays an important role in inflammatory diseases and fibrosis [36].
In case of a negative response to conservative therapy and, as a result, an enlargement of the thyroid gland with significant compression of the trachea and esophagus, surgical treatment (thyroidectomy) is performed. In this case, the frequency of surgical complications (paresis of the recurrent laryngeal nerve, hypoparathyroidism, etc.) approaches 39% of cases, even in expert hands, due to the tight adhesion of the thyroid gland with the surrounding tissues. In such situations, to eliminate the symptoms of compression, they are limited to hemithyroidectomy or removal of the isthmus [5].
CONCLUSION
Elucidation of the common components of the etiopathogenesis of various diseases can significantly improve their diagnosis and treatment, and in the long term — prevention. IgG4-associated endocrine diseases can be considered as an example. Using specialized multimodal (laboratory and instrumental) examination methods, it is possible to quickly and accurately establish the diagnosis, prevalence, clinical prognosis, choose the optimal treatment method and assess the response to it. The prerequisites for this are constant updating of evidence-based knowledge and inter-disciplinary communication. Further in-depth multidisciplinary research is needed to improve diagnostic and treatment algorithms for IgG4-A3 with the involvement of immunologists, endocrinologists, pathologists, radiologists and other specialists.
References
1. Офицеров В.И. Подклассы иммуноглобулина G: возможности использования в диагностической практике: метод. пособие. — Кольцово: Вектор-Бест, 2005. — 19 с. [Ofitserov VI. Podklassy imunoglobulina G: vozmozhnosti ispol'zovaniya v diagnosticheskoy praktike: metod. posobie. Kol'tsovo: Vektor-Best; 2005. 19 р. (In Russ).]
2. Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39(4):469–477. doi: 469-7710.1111/j.1365-2222.2009.03207.x.
3. Deshmukh TM, Shah RR, Gurav YK, Arankalle VA. Serum immunoglobulin G subclass responses in different phases of hepatitis E virus infection. J Med Virol. 2013;85(5):828–832. doi: 10.1002/jmv.23537.
4. Collins AM, Jackson KJ. A temporal model of human IgE and IgG antibody function. Front Immunol. 2013;4:235. doi: 10.3389/fimmu.2013.00235.
5. Rotondi M, Carbone A, Coperchini F, et al. Diagnosis of endocrine disease IgG4-related thyroid autoimmune disease. Eur J Endocrinol. 2019;180(5):R175−R183. doi: 10.1530/EJE-18-1024.
6. Юкина М.Ю., Трошина Е.А., Платонова Н.М., Нуралиева Н.Ф. Аутоиммунная IgG4-ассоциированная эндокринная патология // Ожирение и метаболизм. — 2017. — T.14. — №3. — С. 43–47. [Yukina MYu, Troshina EA, Platonova NM, Nuralieva NF. The autoimmune IgG4 − associated endocrine pathology. Obesity and metabolism. 2017;14(3):43–47. (In Russ).] doi: 10.14341/omet2017343-47.
7. Karim AF, Verdijk RM, Guenoun J, et al. An inflammatory condition with different faces: immunoglobulin G4-related disease. Neth J Med. 2016;74(3):110–115.
8. Vasaitis L. IgG4-related disease: A relatively new concept for clinicians. Eur J Intern Med. 2016;27:1–9. doi: 10.1016/j.ejim.2015.09.022.
9. Ishikawa Y, Terao C. Genetic analysis of IgG4-related disease. Mod Rheumatol. 2019;30(1):17–23. doi: 10.1080/14397595.2019.1621000.
10. Morales A, Cignarella A, Jabeen IS, et al. An update on IgG4-related lung disease. Eur J Intern Med. 2019;66:18–24. doi: 10.1016/j.ejim.2019.06.010.
11. Wallace ZS, Zhang Y, Perugino CA, et al. Clinical phenotypes of IgG4-related disease: an analysis of two international cross-sectional cohorts. Ann Rheum Dis. 2019;78(3):406–412. doi: 10.1136/annrheumdis-2018-214603.
12. Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4- related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22(1):21–30. doi: 10.1007/s10165-011-0571-z.
13. Chen H, Lin W, Wang Q, et al. IgG4-related disease in a Chinese cohort: a prospective study. Scand J Rheumatol. 2014;43(1):70–74. doi: 10.3109/ 03009742.2013.822094.
14. Wallace ZS, Naden RP, Chari S, et al. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG -related disease. Ann Rheum Dis. 2020;79(1):77–87. doi: 10.1136/ annrheumdis-2019-216561.
15. Masaki Y, Kurose N, Yamamoto M, et al. Cutoff values of serum IgG4 and histopathological IgG4+ plasma cells for diagnosis of patients with IgG4-related disease. Int J Rheumatol. 2012:580814. doi: 10.1155/2012/580814.
16. Hao M, Liu M, Fan G, et al. Diagnostic value of serum IgG4 for IgG4-related disease: a PRISMA-compliant systematic review and Meta-analysis. Medicine (Baltimore). 2016;95(21):e3785. doi: 10.1097/MD.0000000000003785.
17. Culver EL, Sadler R, Simpson D, et al. Elevated serum IgG4 levels in diagnosis, treatment response, organ involvement, and relapse in a prospective IgG4-related disease UK cohort. Am J Gastroenterol. 2016;111(5):733–743. doi: 10.1038/ajg.2016.40.
18. Tang J, Cai S, Ye C, et al. Biomarkers in IgG4-related disease: a systematic review. Semin Arthritis Rheum. 2020;50(2):354–359. doi: 10.1016/j.semarthrit.2019.06.018.
19. Akiyama M, Yasuoka H, Yoshimoto K, et al. CC-chemokine ligand 18 is a useful biomarker associated with disease activity in IgG4-related disease. Ann Rheum Dis. 2018;77(9):1386–1387. doi: 10.1136/annrheumdis-2017-212110.
20. Furukawa S, MoriyamaM, Tanaka A, et al. Preferential M2 macrophages contribute to fibrosis in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz’s disease. Clin Immunol. 2015;156(1):9–18. doi: 10.1016/j.clim.2014.10.008.
21. Shiokawa M, Kodama Y, Sekiguchi K, et al. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci Transl Med. 2018;10(453):eaaq0997. doi: 10.1126/scitranslmed.aaq0997.
22. Della-Torre E, Rigamonti E, Perugino C, et al. B lymphocytes directly contribute to tissue fibrosis in patients with IgG4-related disease J Allergy Clin Immunol. doi: 10.1016/j.jaci.2019.07.004.
23. Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-Related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190–195. doi: 10.1136/annrheumdis-2014-205233.
24. Lanzillotta M, Della-Torre E, Milani R, et al. Effects of glucocorticoids on B-cell subpopulations in patients with IgG4-related disease. Clin Exp Rheumatol. 2019;37Suppl 118(3):159–166.
25. Kamisawa T, Zen Y, Pillai S, et al. IgG4-related disease. Lancet. 2015;385(9976):1460–1471. doi: 10.1016/S0140-6736(14)60720-0.
26. Giesel FL, Heussel CP, Lindner T, et al. FAPI-PET/CT improves staging in a lung cancer patient with cerebral metastasis. Eur J Nucl Med Mol Imaging. 2019;46(8):1754–1755. doi: 10.1007/s00259-019-04346-z.
27. Kratochwil C, Flechsig P, Lindner T, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 Different kinds of cancer. J Nucl Med. 2019;60(6):801–805. doi: 10.2967/jnumed.119.227967.
28. Luo Y, Pan Q, Zhang W. IgG4-related disease revealed by 68Ga-FAPI and 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2019;46(12):2625–2626. doi: 10.1007/s00259-019-04478-2.
29. Седышев С.Х., Васильев В.И., Ковригина А.М., Насонов Е.Л. Igg4-связанное системное заболевание. Современный взгляд на «Старые» болезни // Научно-практическая ревматология. — 2012. — №5. — С. 64–72. [Sedyshev SKh, Vasiliev VI, Kovriguina AM, Nasonov EL. Igg4-linked systemic disease. Modern outlook on «Old» disease. Nauchno-prakticheskaia revmatologiia. 2012;(5):64–72. (In Russ).]
30. Kurowecki D, Patlas MN, Haider EA, et al. Cross-sectional pictorial review of IgG4-related disease. Br J Radiol. 2019;92(1103):20190448. doi: 10.1259/bjr.20190448.
31. Dean T. Jeffery, Hillary R. Kelly, 40 - IgG4-Related Disease in the Head and Neck, Neuroradiology, 2019, Pages 308-317, ISBN 9780323445498, https://doi.org/10.1016/B978-0-323-44549-8.00040-7.
32. Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-Related disease. Arthritis Rheumatol. 2015;67(7):1688–1699. doi: 10.1002/art.39132.
33. Ahn C, Kang S, Sa HS. Clinicopathologic features of biopsied lacrimal gland masses in 95 Korean patients. Graefes Arch Clin Exp Ophthalmol. 2019;257(7):1527–1533. doi: 10.1007/s00417-019-04327-w.
34. Omar D, Chen Y, Cong Y, et al. Glucocorticoids and steroid sparing medications monotherapies or in combination for IgG4-RD: a systematic review and network meta-analysis. Rheumatology (Oxford). 2020;59(4):718–726. doi: 10.1093/rheumatology/kez380.
35. Сокол Е.В., Васильев В.И. Лечение IgG4-связанного заболевания // Научно-практическая ревматология. — 2016. — T.54. — №3. — С. 352–360. [Sokol EV, Vasilyev VI. Treatment of IgG4-related disease. Nauchno-prakticheskaia revmatologiia. 2016;54(3):352–360. (In Russ).] doi: 10.14412/1995-4484-2016-352-360.
36. Watanabe T, Minaga K, Kamata K, et al. Mechanistic insights into autoimmune pancreatitis and IgG4-Related disease. Trends Immunol. 2018;39(11):874–889. doi: 10.1016/j.it.2018.09.005.
About the Authors
Pavel O. RumyantsevRussian Federation
MD, PhD
Ivan G. Kozlov
Russian Federation
MD, PhD
Evgenia A. Kolpakova
Russian Federation
Resident
Olga S. Chukhacheva
Russian Federation
MD
Sergey V. Korenev
Russian Federation
MD, PhD
Andrei G. Goncharov
Russian Federation
MD, PhD
Elena U. Ulanova
Russian Federation
Head teacher
Supplementary files
|
|
1. Рисунок 1. Варианты клинической манифестации IgG4-АЗ. Picture 1. Clinical manifestations of IgG4-RD. | |
| Subject | ||
| Type | Other | |
View
(96KB)
|
Indexing metadata ▾ | |
|
|
2. Рисунок 2. Схема иммунопатогенеза IgG4-АЗ. Picture 2. Immunopathogenesis scheme of IgG4-RD. | |
| Subject | ||
| Type | Other | |
View
(326KB)
|
Indexing metadata ▾ | |
|
|
3. Рисунок 3. Алгоритм диагностики IgG4-АЗ. Picture 3. Diagnostic algorithm of IgG4-RD. | |
| Subject | ||
| Type | Other | |
View
(118KB)
|
Indexing metadata ▾ | |
|
|
4. Рисунок 4. Предполагаемые биомаркеры IgG4-АЗ. Picture 4. Supposed biomarkers of IgG4-RD. | |
| Subject | ||
| Type | Other | |
View
(55KB)
|
Indexing metadata ▾ | |
Review
For citations:
Rumyantsev P.O., Kozlov I.G., Kolpakova E.A., Chukhacheva O.S., Korenev S.V., Goncharov A.G., Ulanova E.U. IGG4-related diseases in endocrinology. Problems of Endocrinology. 2020;66(2):24-32. https://doi.org/10.14341/probl12285

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0).









































.jpg)


